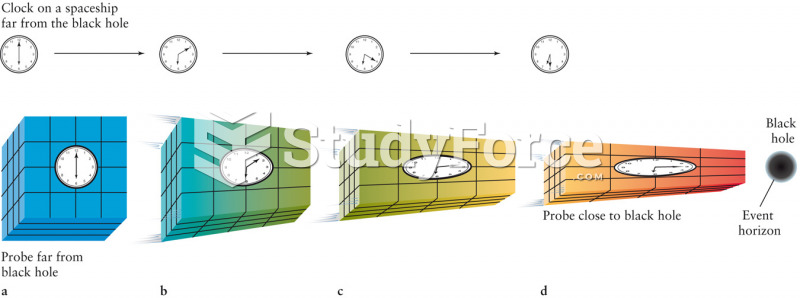
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Did you know?
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.