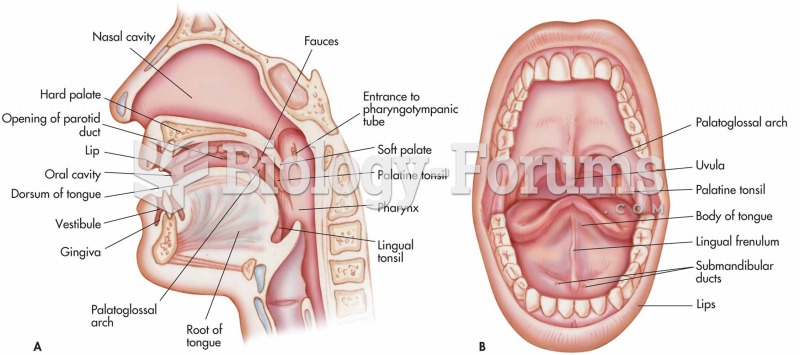
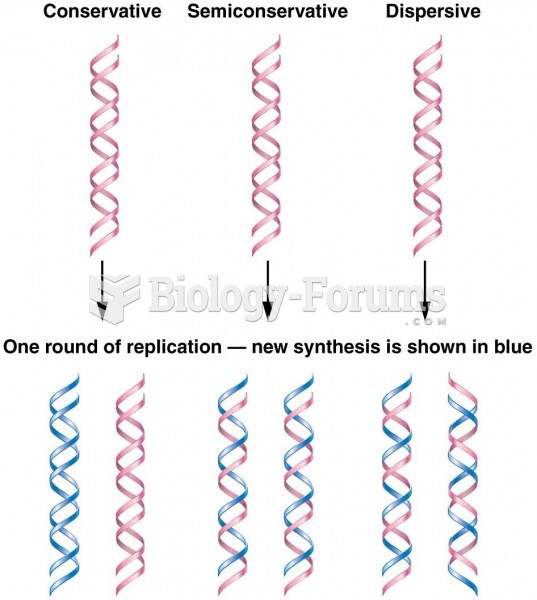
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
Did you know?
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
Did you know?
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
Did you know?
The word drug comes from the Dutch word droog (meaning "dry"). For centuries, most drugs came from dried plants, hence the name.