|
|
|
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Liver spots have nothing whatsoever to do with the liver. They are a type of freckles commonly seen in older adults who have been out in the sun without sufficient sunscreen.
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
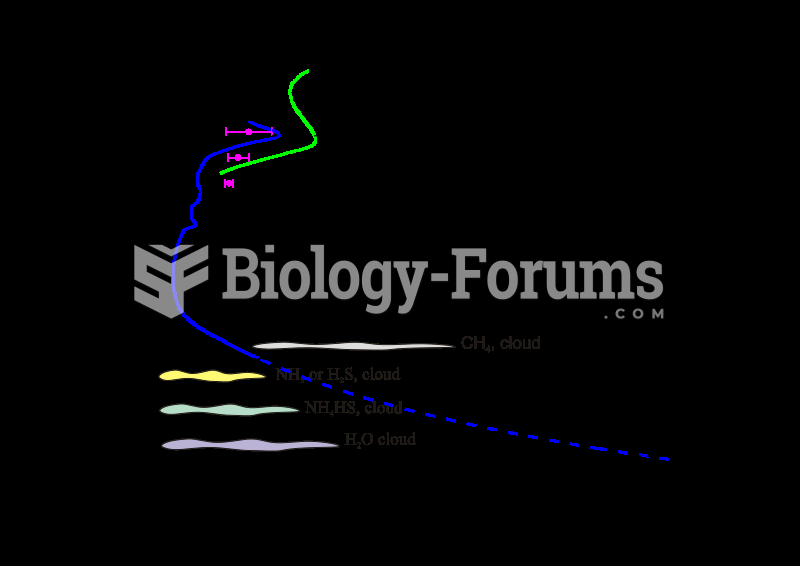 Temperature profile of the Uranian troposphere and lower stratosphere. Cloud and haze layers are als
Temperature profile of the Uranian troposphere and lower stratosphere. Cloud and haze layers are als
 Localization of a Peptide The peptide is revealed by means of immunocytochemistry. The photomicrogra
Localization of a Peptide The peptide is revealed by means of immunocytochemistry. The photomicrogra
 Chinese workers on a railway in the far West. “Without them,” Leland Stanford, president of the Cent
Chinese workers on a railway in the far West. “Without them,” Leland Stanford, president of the Cent