|
|
|
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
There are approximately 3 million unintended pregnancies in the United States each year.
There used to be a metric calendar, as well as metric clocks. The metric calendar, or "French Republican Calendar" divided the year into 12 months, but each month was divided into three 10-day weeks. Each day had 10 decimal hours. Each hour had 100 decimal minutes. Due to lack of popularity, the metric clocks and calendars were ended in 1795, three years after they had been first marketed.
The word drug comes from the Dutch word droog (meaning "dry"). For centuries, most drugs came from dried plants, hence the name.
 Approximately 1 pound (0.45 kg) of ground elk meat formed into patties; note the relatively small fa
Approximately 1 pound (0.45 kg) of ground elk meat formed into patties; note the relatively small fa
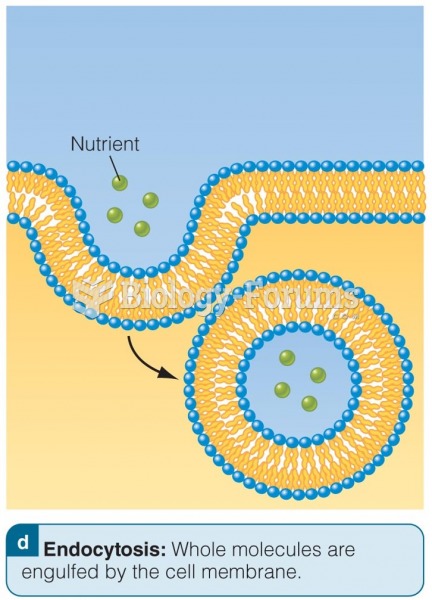
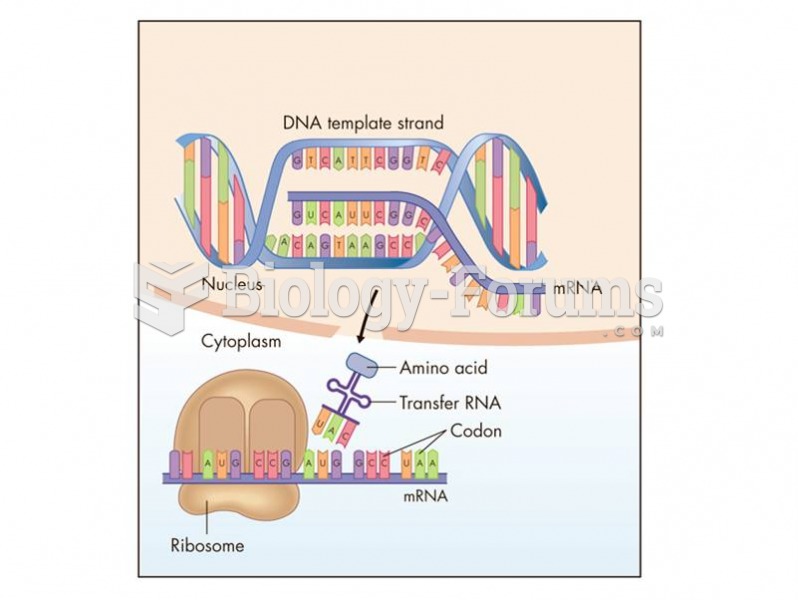 Messenger RNA (mRNA) carries genetic information from the nucleus to the cytoplasm for protein synth
Messenger RNA (mRNA) carries genetic information from the nucleus to the cytoplasm for protein synth