|
|
|
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
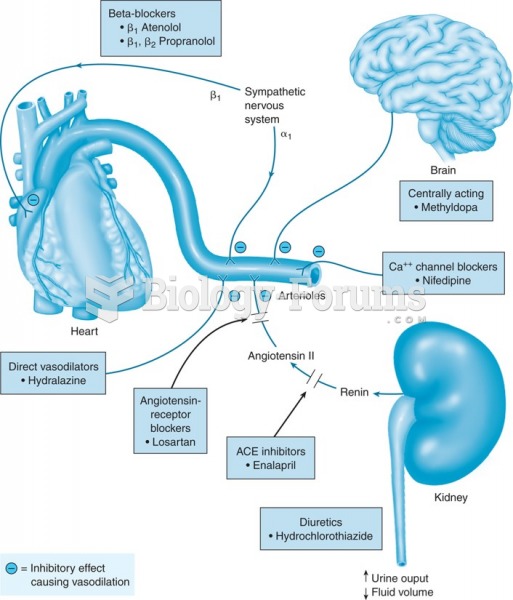
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
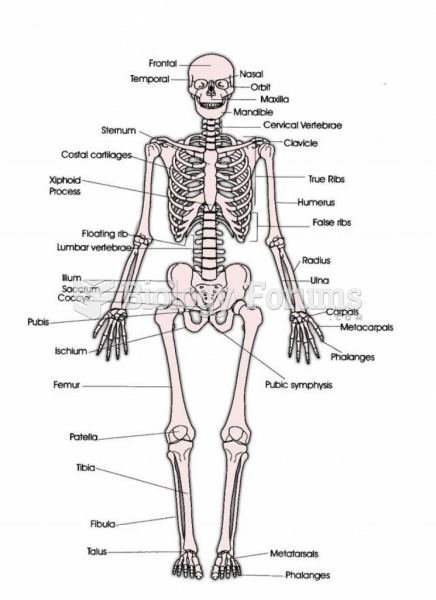
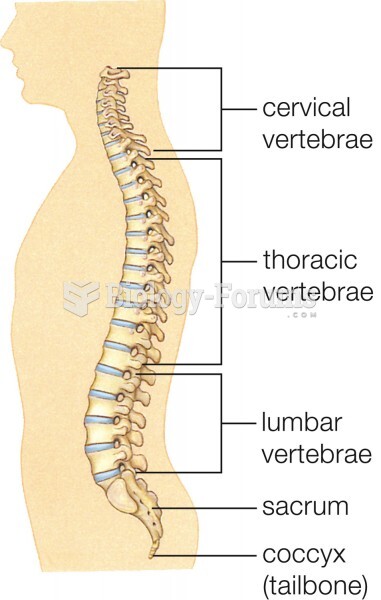
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.
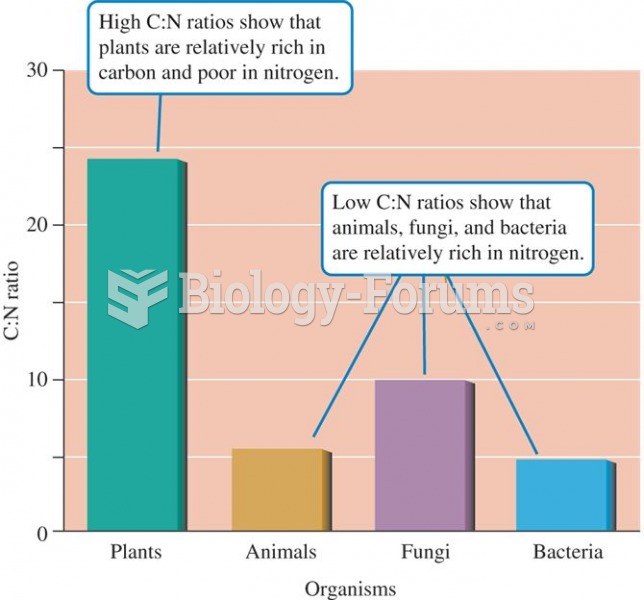 On average, the ratio of carbon to nitrogen is much higher in terrestrial plants than in other major
On average, the ratio of carbon to nitrogen is much higher in terrestrial plants than in other major
 This red gasoline container holds about 30 gallons of gasoline and is used to fill vehicles used ...
This red gasoline container holds about 30 gallons of gasoline and is used to fill vehicles used ...