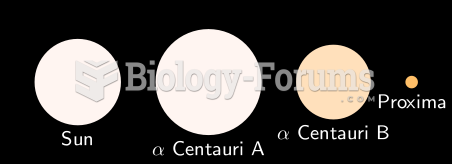
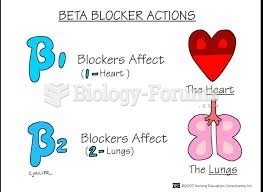
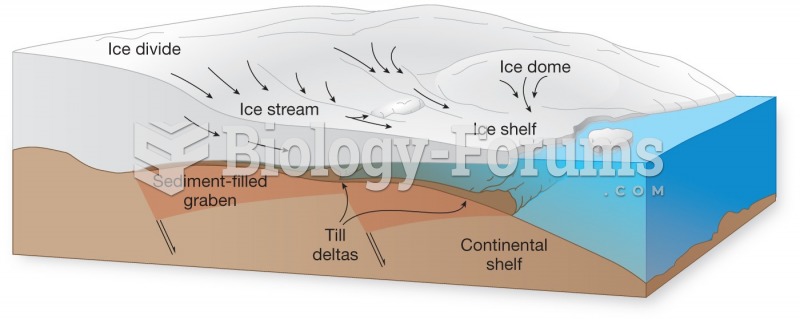
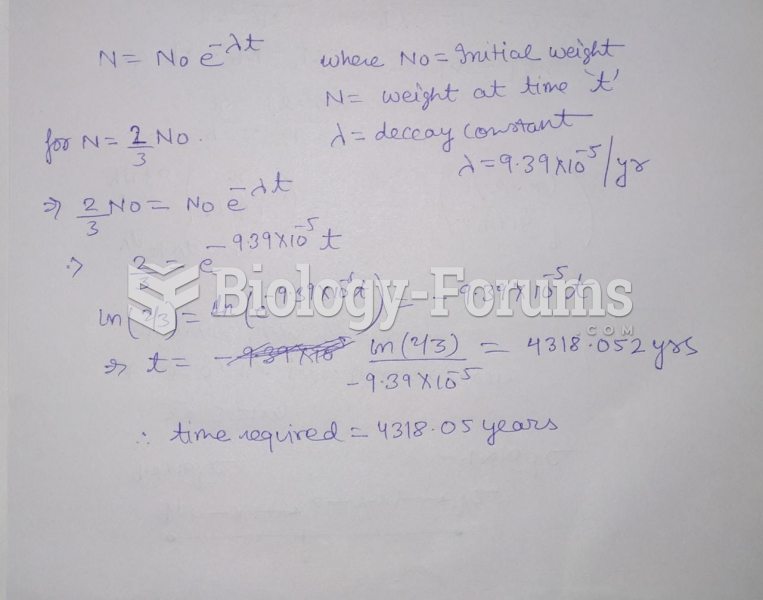
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
Did you know?
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.