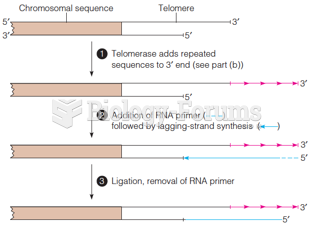
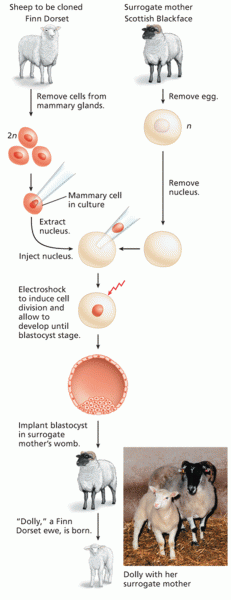
|
|
|
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.