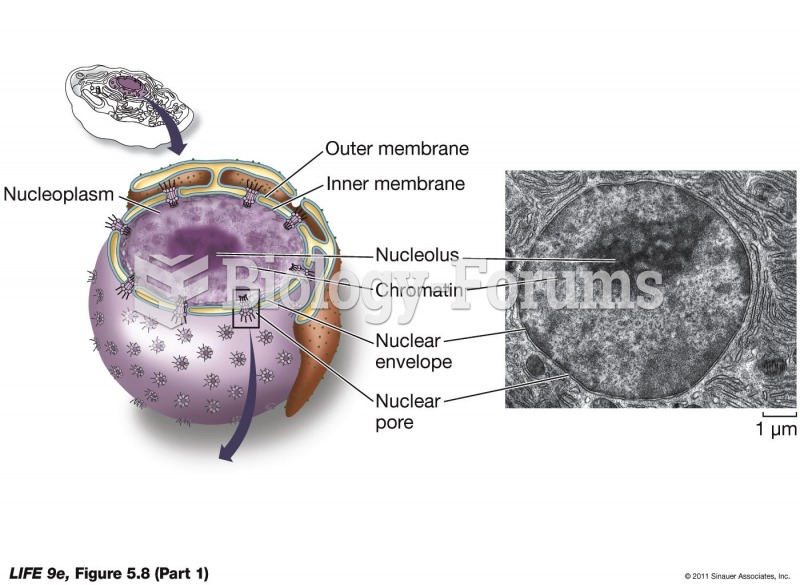
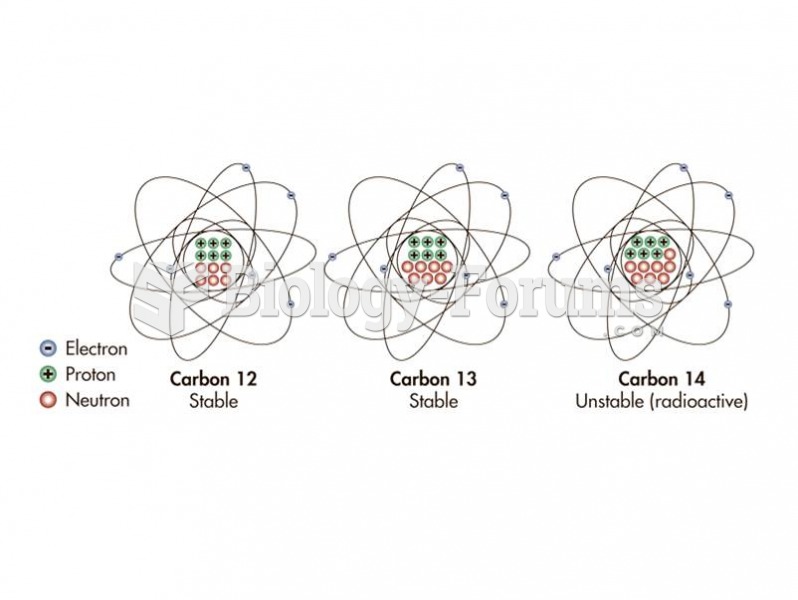
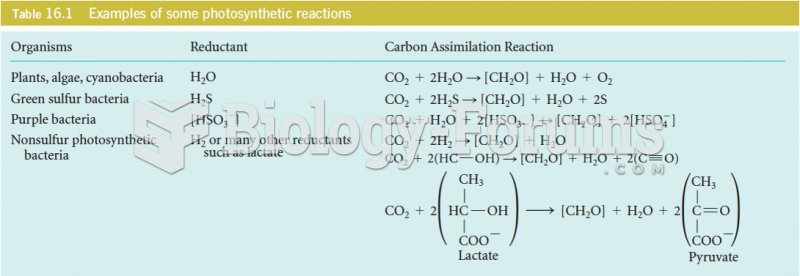
|
|
|
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.
According to the American College of Allergy, Asthma & Immunology, more than 50 million Americans have some kind of food allergy. Food allergies affect between 4 and 6% of children, and 4% of adults, according to the CDC. The most common food allergies include shellfish, peanuts, walnuts, fish, eggs, milk, and soy.
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
There are 60,000 miles of blood vessels in every adult human.
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
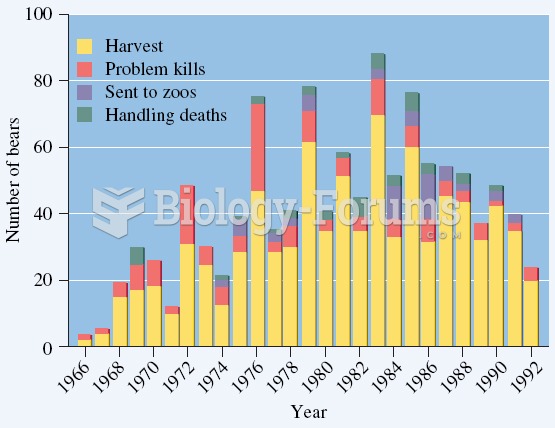 Over nearly 30 years, humans removed many polar bears for a variety of reasons, from a population in
Over nearly 30 years, humans removed many polar bears for a variety of reasons, from a population in
 Bow shock formed by the magnetosphere of LL Orionis (center) as it collides with the Orion Nebula fl
Bow shock formed by the magnetosphere of LL Orionis (center) as it collides with the Orion Nebula fl