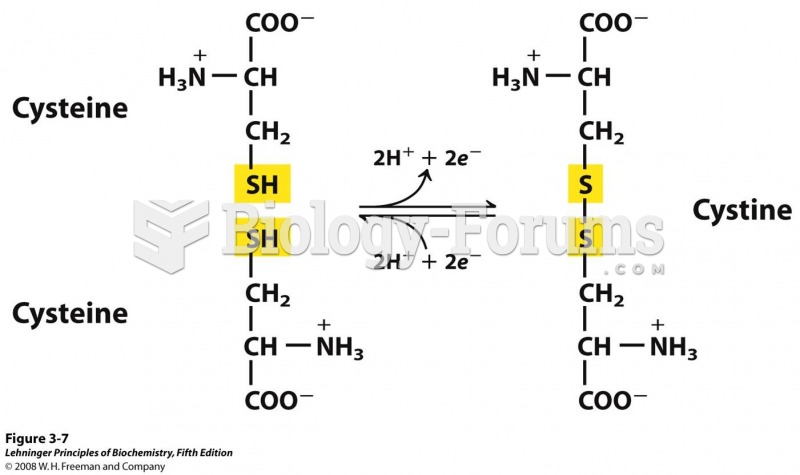
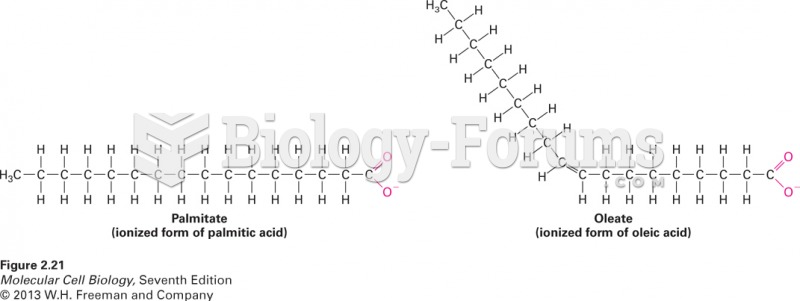
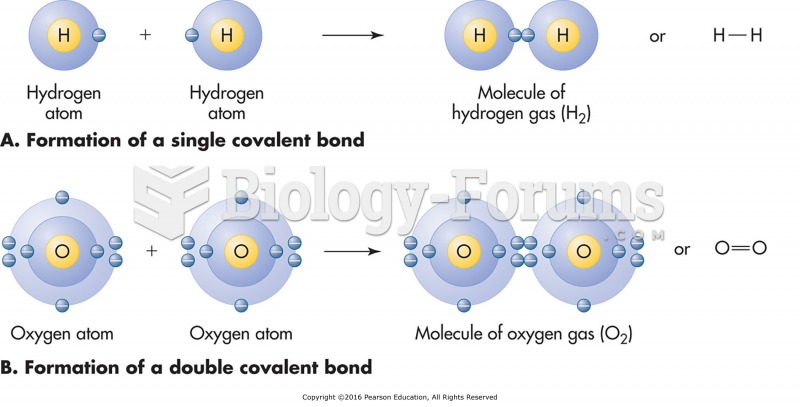
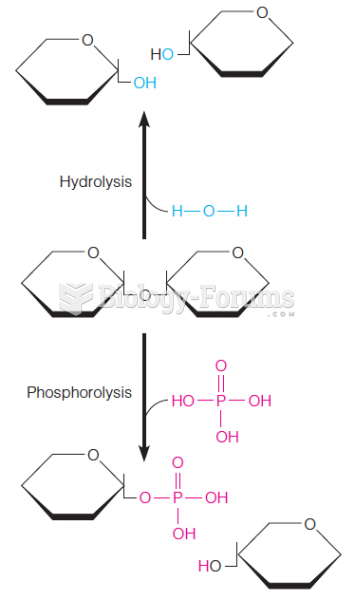
|
|
|
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
Anesthesia awareness is a potentially disturbing adverse effect wherein patients who have been paralyzed with muscle relaxants may awaken. They may be aware of their surroundings but unable to communicate or move. Neurologic monitoring equipment that helps to more closely check the patient's anesthesia stages is now available to avoid the occurrence of anesthesia awareness.
The average office desk has 400 times more bacteria on it than a toilet.