This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
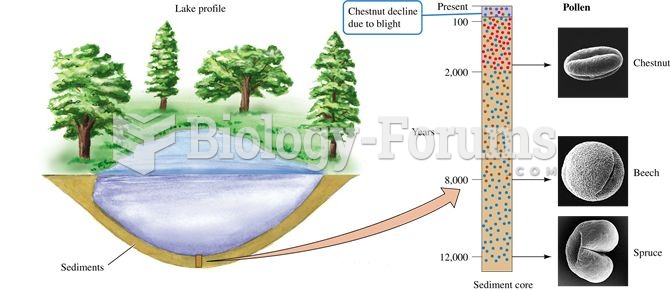 The vegetation history of landscapes can be reconstructed using the pollen contained within the sedi
The vegetation history of landscapes can be reconstructed using the pollen contained within the sedi
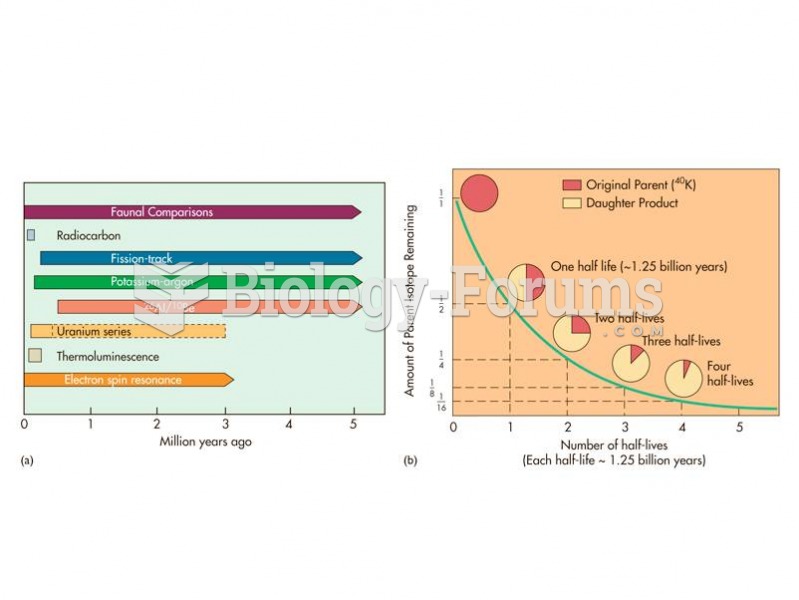 (a) The relative age ranges of different dating techniques depend upon the half-life of the system u
(a) The relative age ranges of different dating techniques depend upon the half-life of the system u