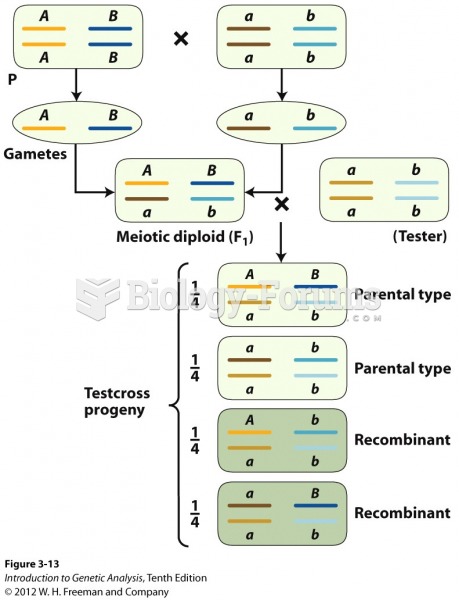
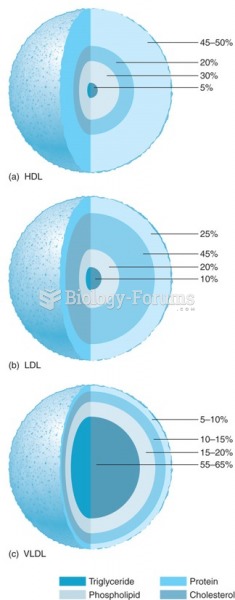
|
|
|
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.