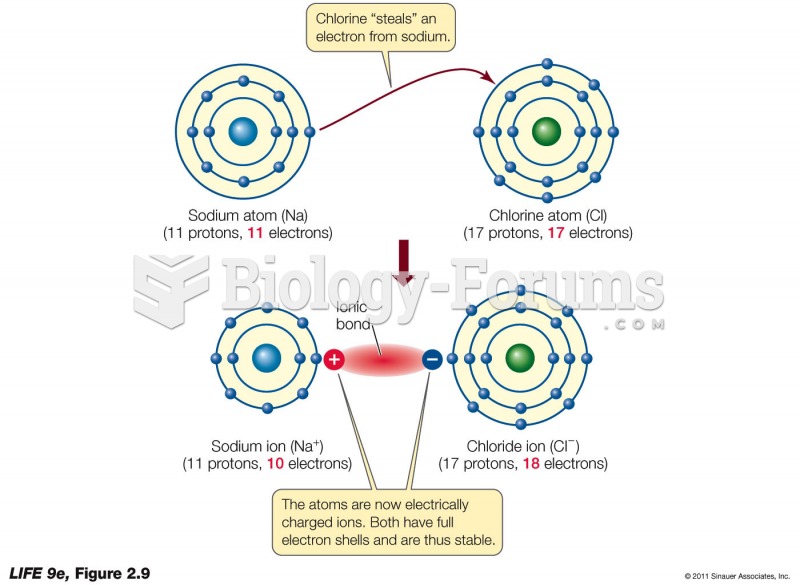
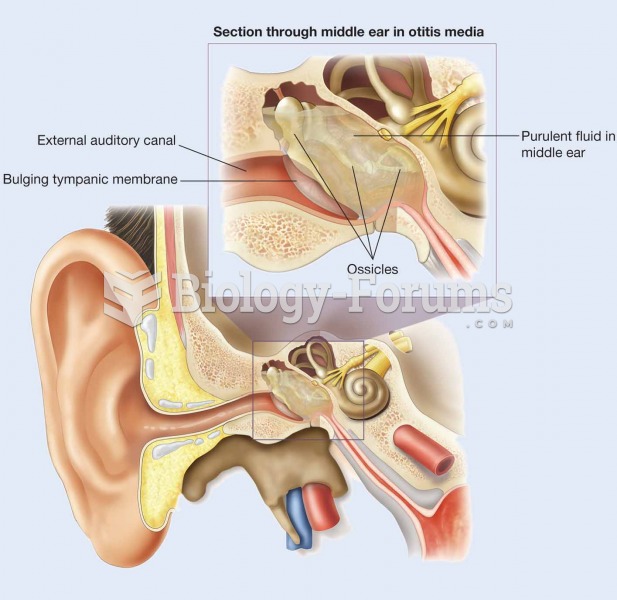
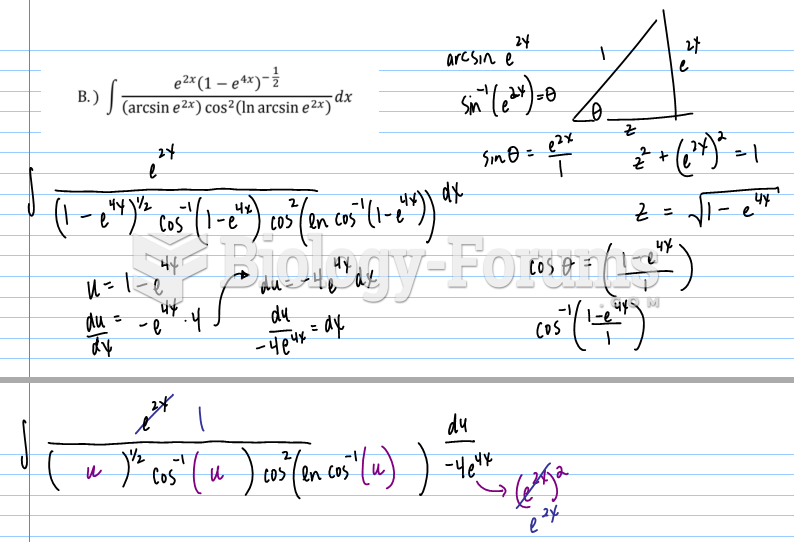
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Did you know?
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.