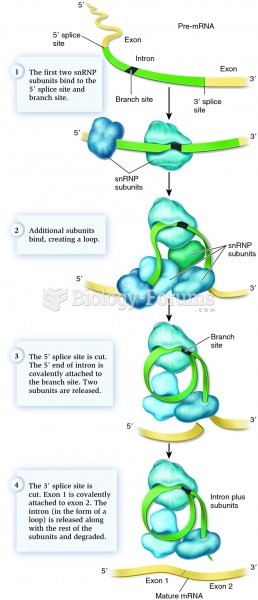
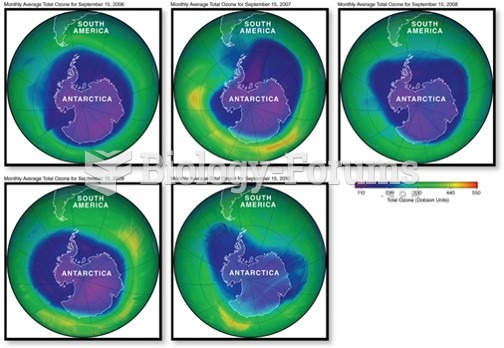
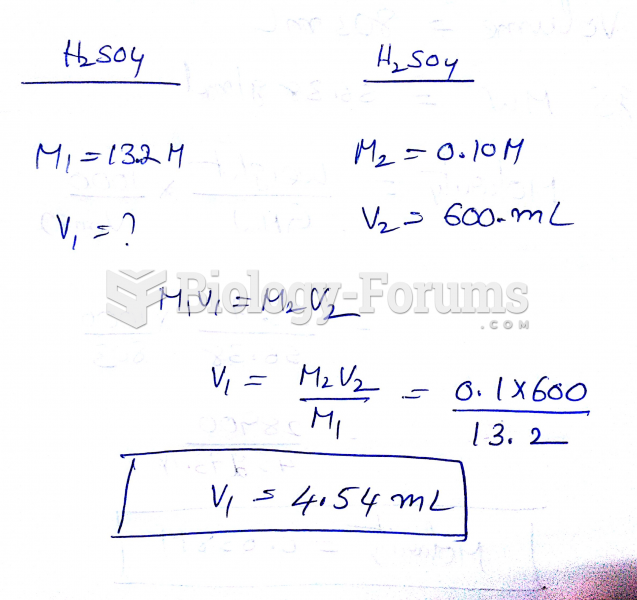
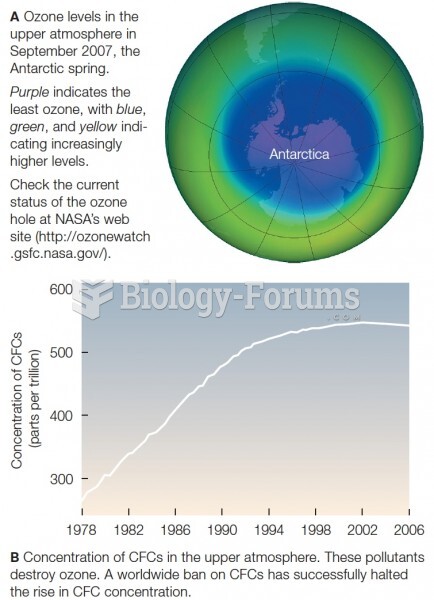
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.