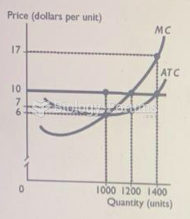
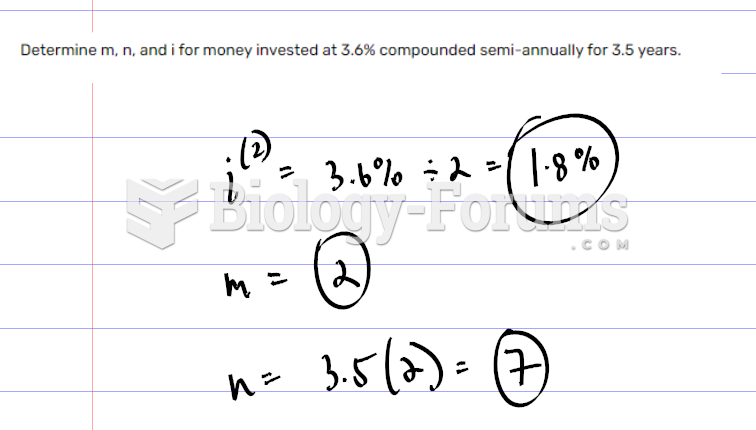
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
Ether was used widely for surgeries but became less popular because of its flammability and its tendency to cause vomiting. In England, it was quickly replaced by chloroform, but this agent caused many deaths and lost popularity.
Did you know?
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
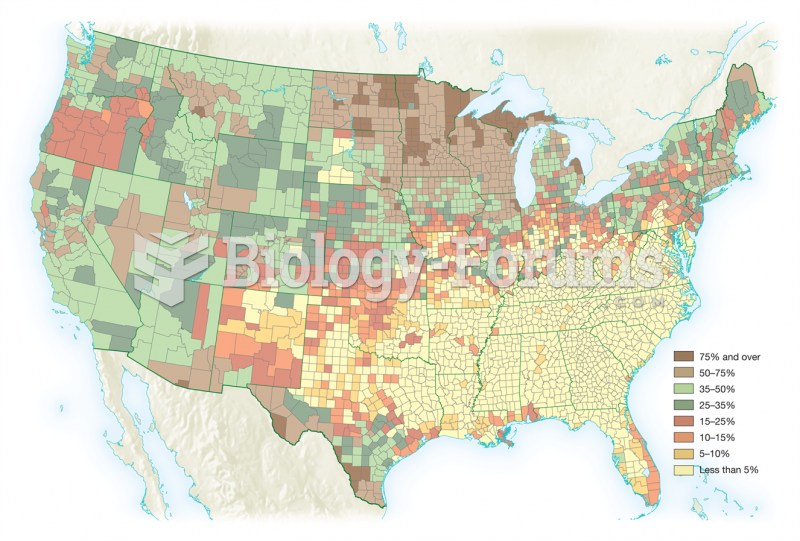
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.