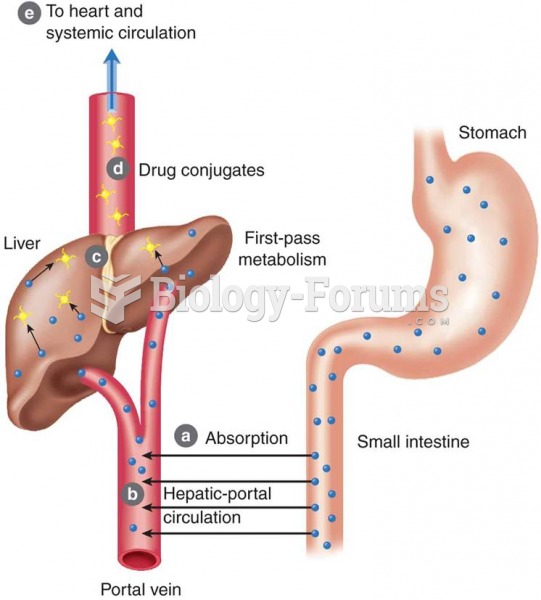
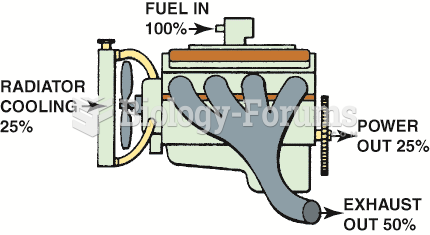
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
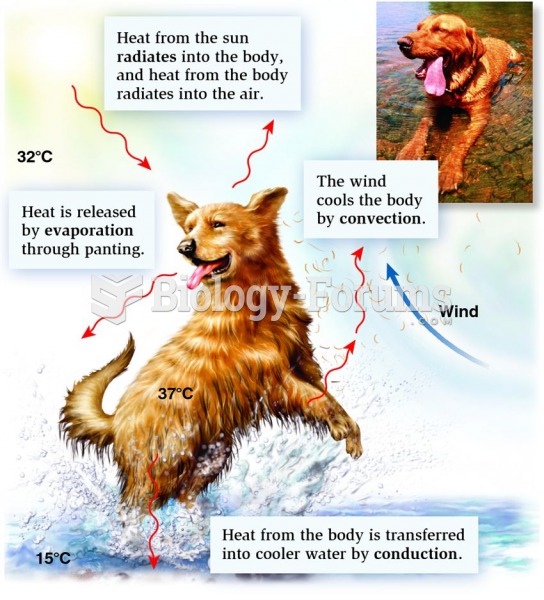 The four ways in which animals exchange heat with the environment are radiation, evaporation, convec
The four ways in which animals exchange heat with the environment are radiation, evaporation, convec
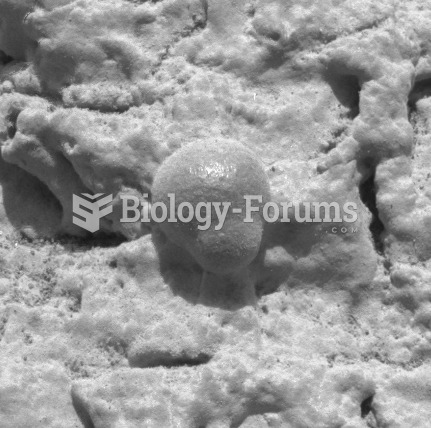 Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr
Microscopic photo taken by Opportunity showing a gray hematite concretion, indicative of the past pr