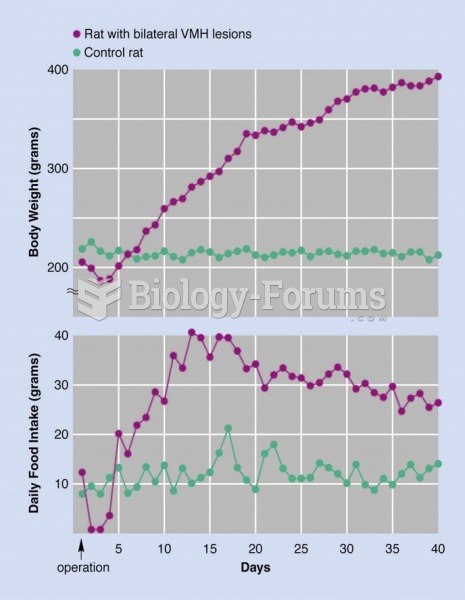
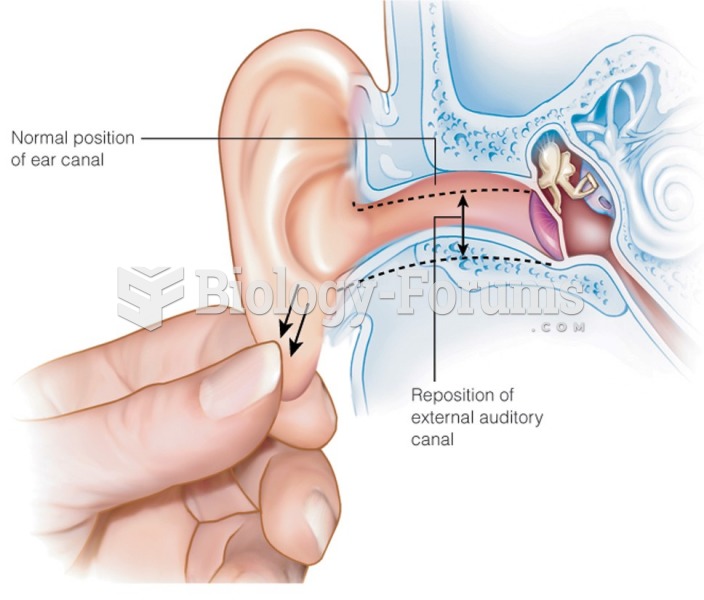
|
|
|
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
If you use artificial sweeteners, such as cyclamates, your eyes may be more sensitive to light. Other factors that will make your eyes more sensitive to light include use of antibiotics, oral contraceptives, hypertension medications, diuretics, and antidiabetic medications.
The Food and Drug Administration has approved Risperdal, an adult antipsychotic drug, for the symptomatic treatment of irritability in children and adolescents with autism. The approval is the first for the use of a drug to treat behaviors associated with autism in children. These behaviors are included under the general heading of irritability and include aggression, deliberate self-injury, and temper tantrums.
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Green tea is able to stop the scent of garlic or onion from causing bad breath.
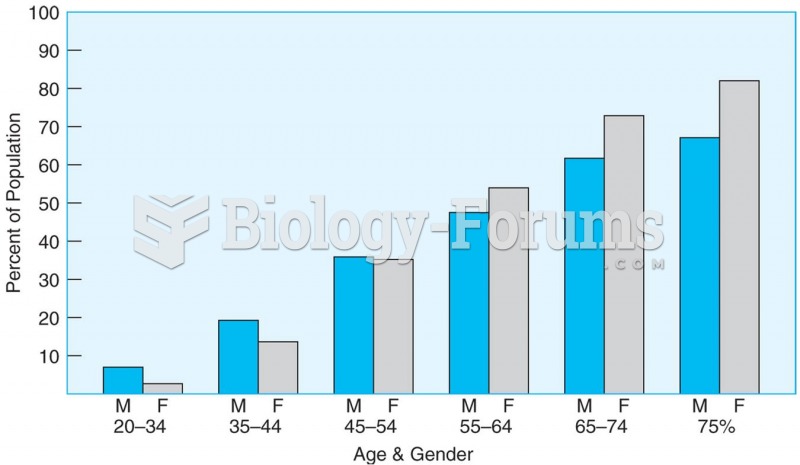 The proportion of men and women with elevated blood pressure or taking hypertension medication incre
The proportion of men and women with elevated blood pressure or taking hypertension medication incre
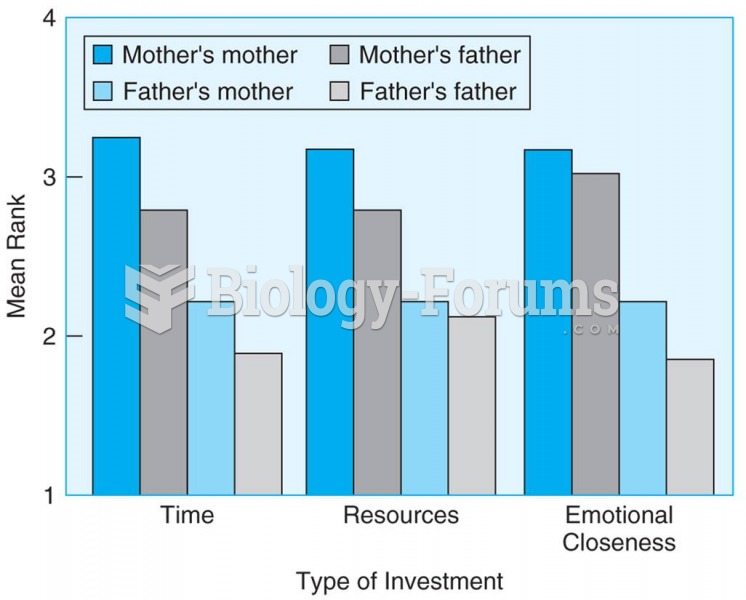 Students rated their grandparents on a scale from 1 to 4 based on emotional closeness, time spent ...
Students rated their grandparents on a scale from 1 to 4 based on emotional closeness, time spent ...