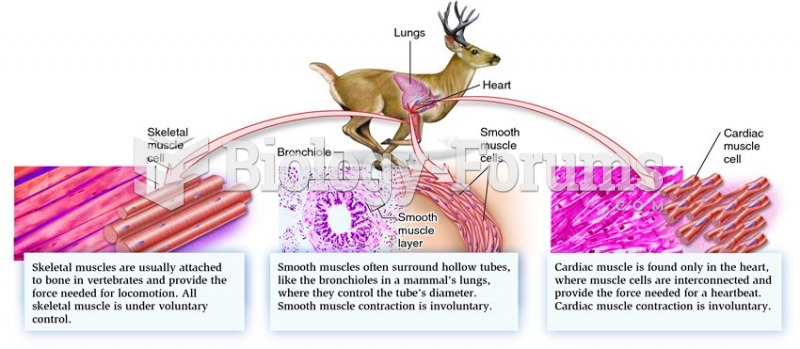
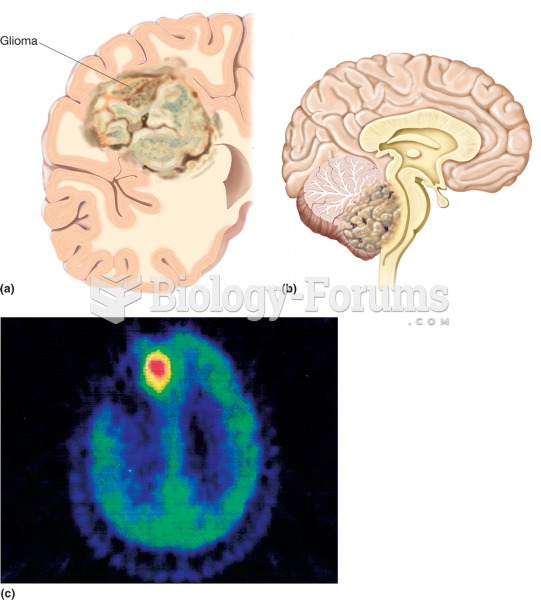
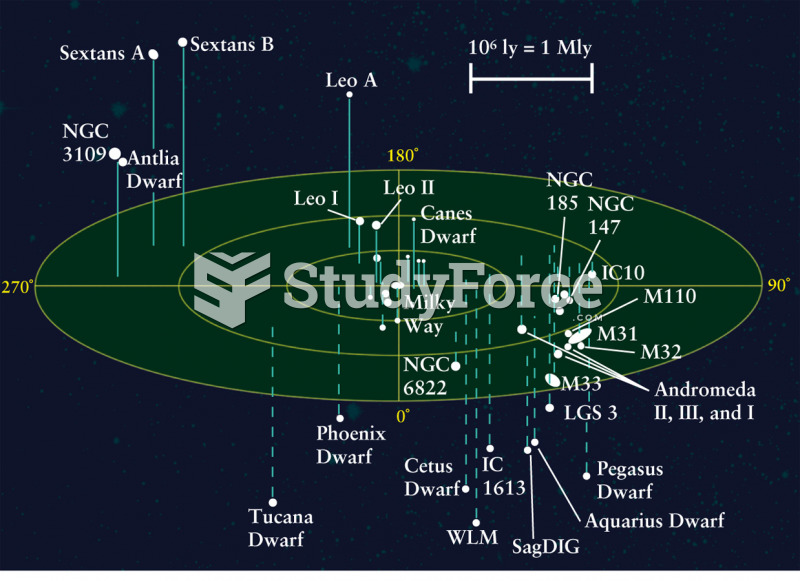
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Your heart beats over 36 million times a year.