|
|
|
Approximately 25% of all reported medication errors result from some kind of name confusion.
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
Only 12 hours after an egg cell is fertilized by a sperm cell, the egg cell starts to divide. As it continues to divide, it moves along the fallopian tube toward the uterus at about 1 inch per day.
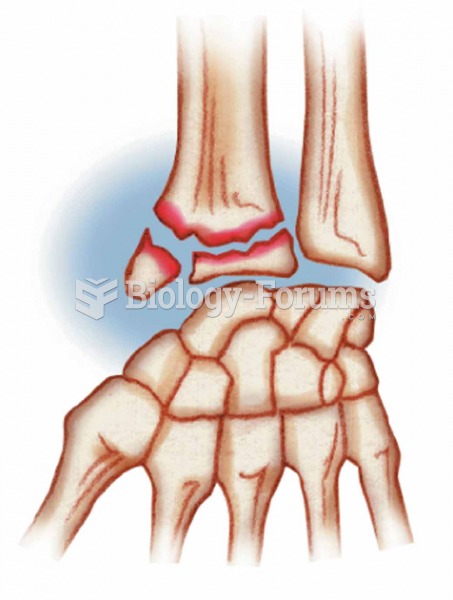 Colles’ A break in the distal portion of the radius; often the result of reaching out to cushion a f
Colles’ A break in the distal portion of the radius; often the result of reaching out to cushion a f
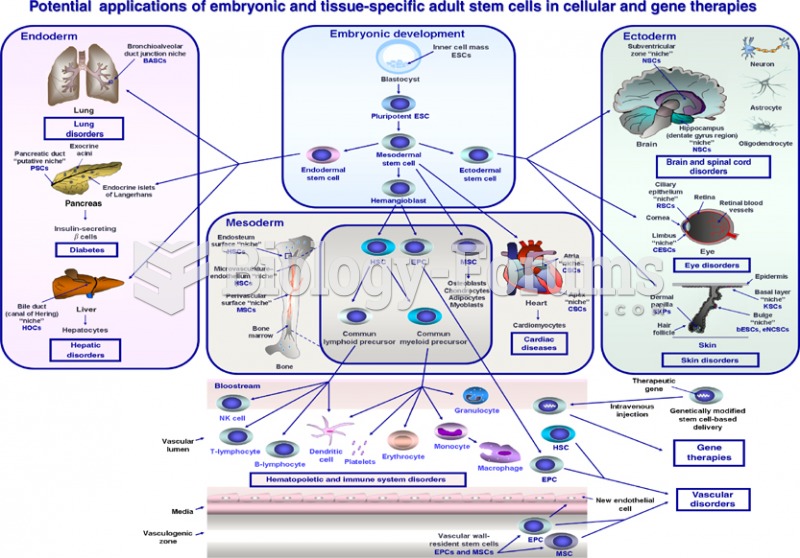 Potential applications of embryonic and tissue specific adult stem cell in cellular and gene theraph
Potential applications of embryonic and tissue specific adult stem cell in cellular and gene theraph