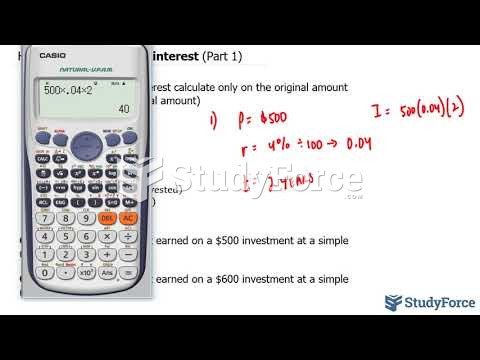
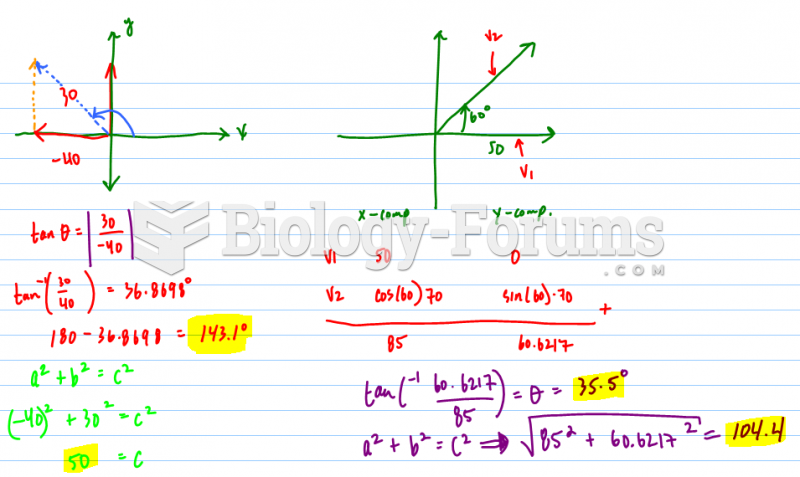
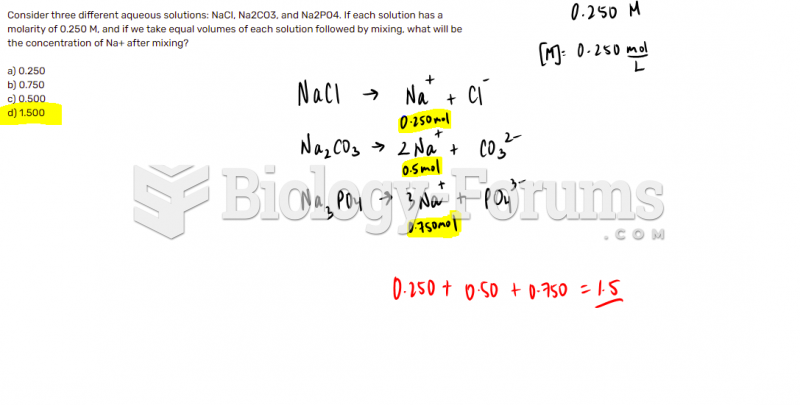
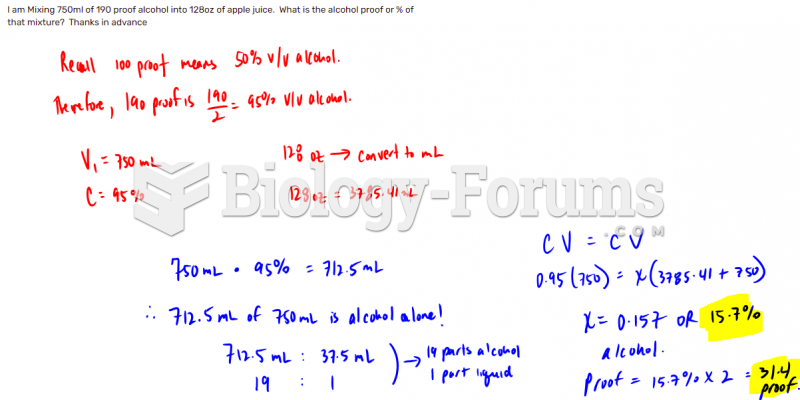
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.