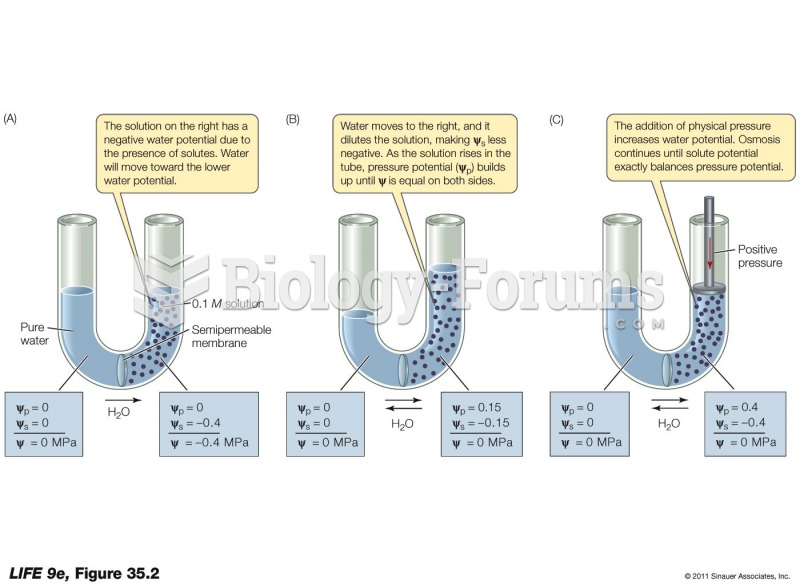
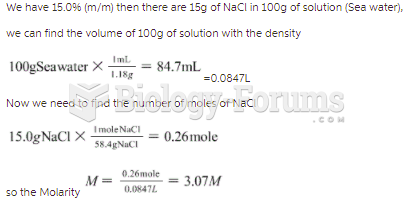This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.