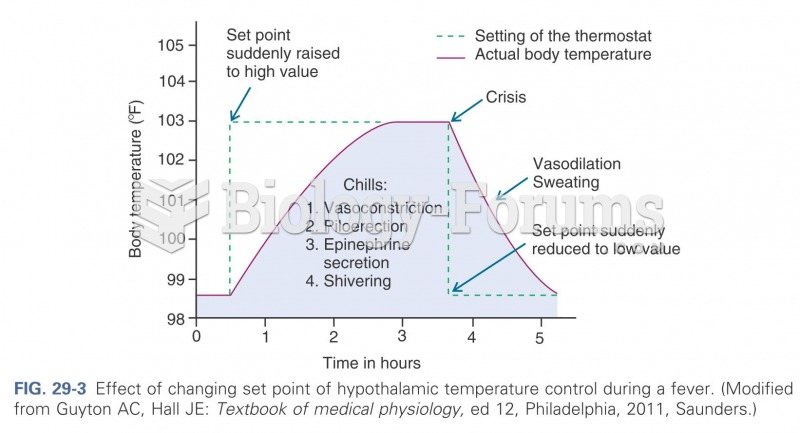
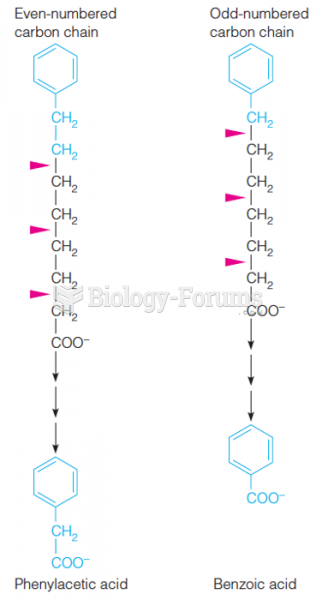
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
Did you know?
Addicts to opiates often avoid treatment because they are afraid of withdrawal. Though unpleasant, with proper management, withdrawal is rarely fatal and passes relatively quickly.
Did you know?
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.