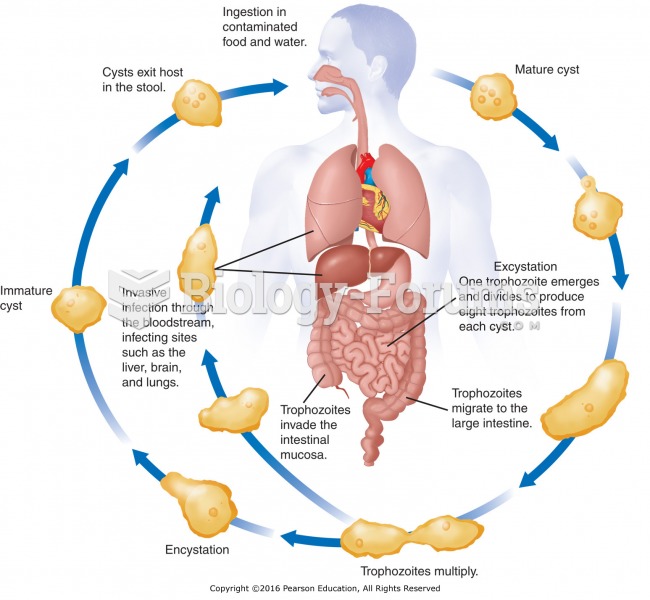
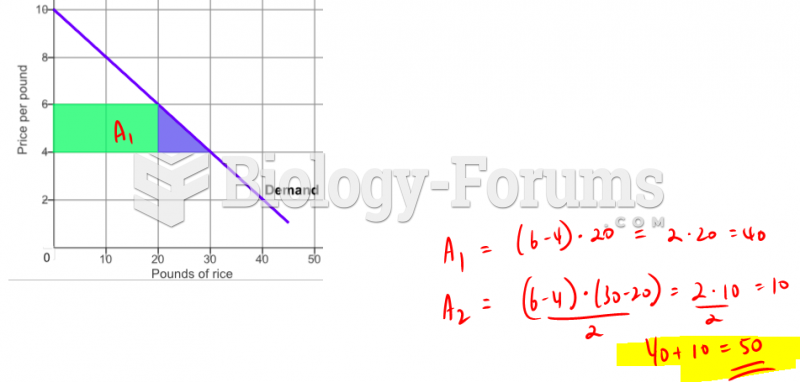
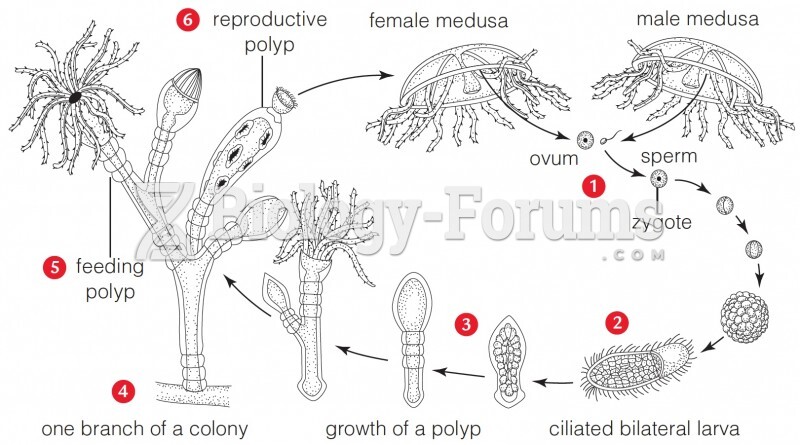
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.