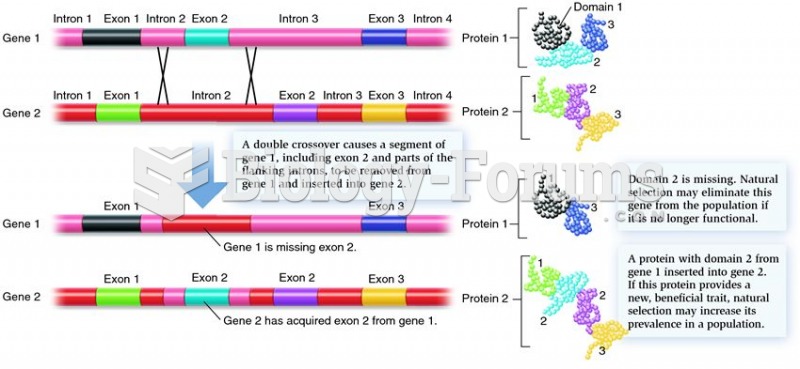
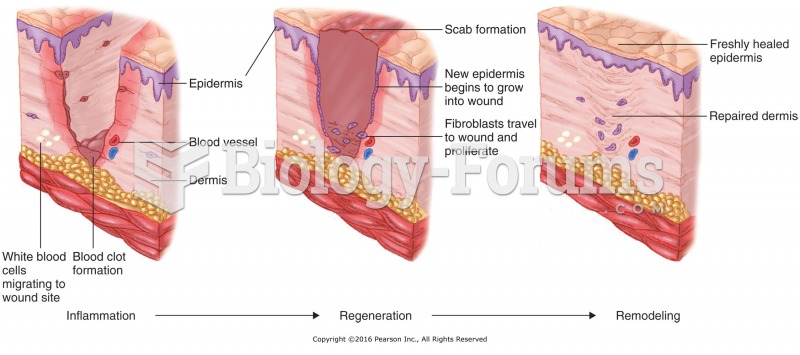
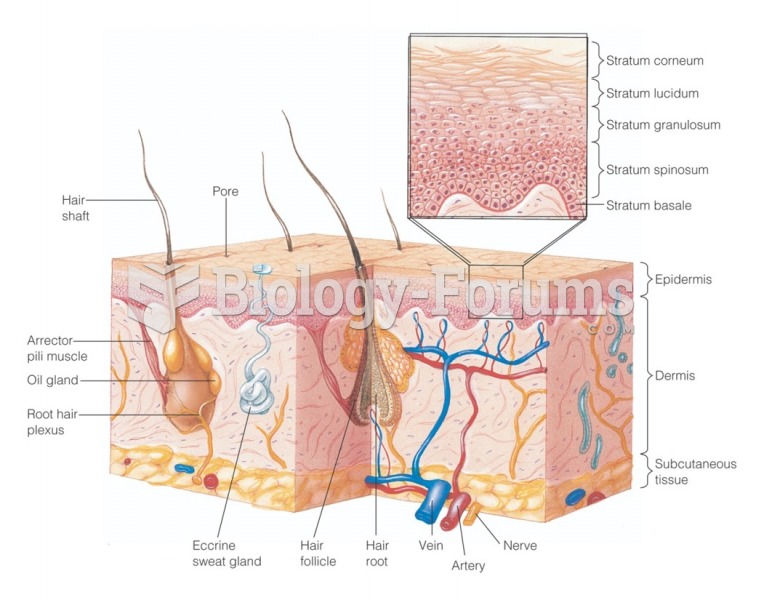
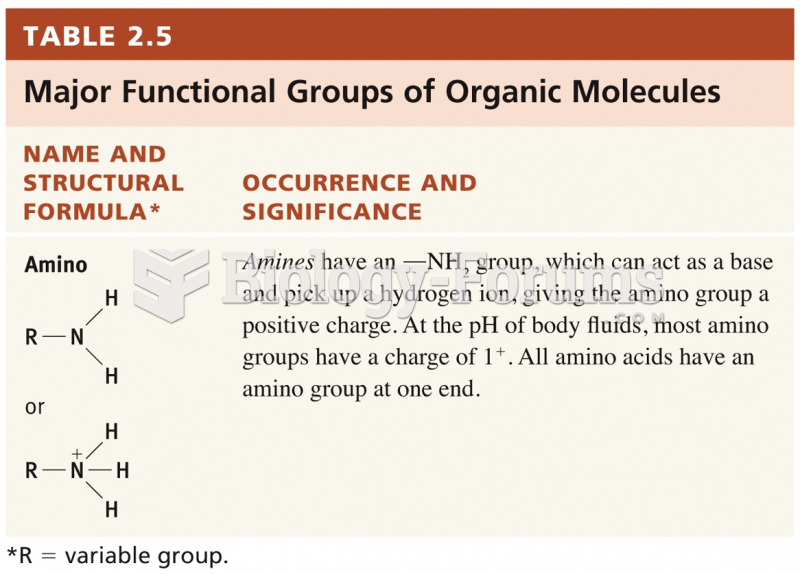
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
To maintain good kidney function, you should drink at least 3 quarts of water daily. Water dilutes urine and helps prevent concentrations of salts and minerals that can lead to kidney stone formation. Chronic dehydration is a major contributor to the development of kidney stones.
Did you know?
When taking monoamine oxidase inhibitors, people should avoid a variety of foods, which include alcoholic beverages, bean curd, broad (fava) bean pods, cheese, fish, ginseng, protein extracts, meat, sauerkraut, shrimp paste, soups, and yeast.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
Cyanide works by making the human body unable to use oxygen.