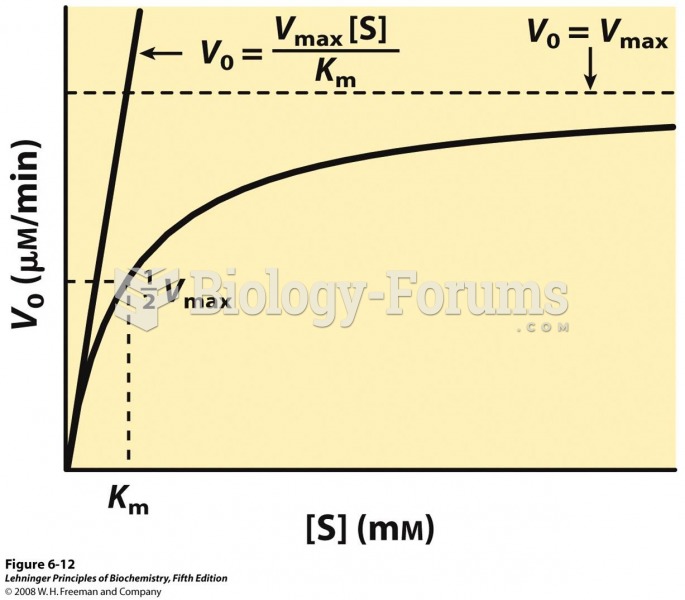
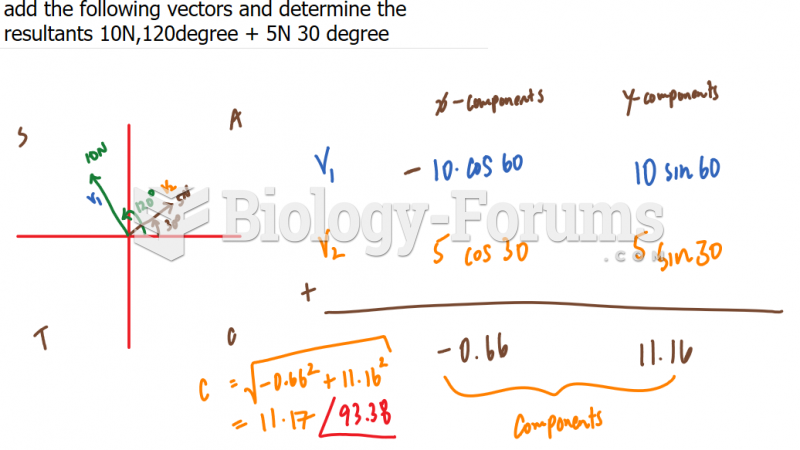
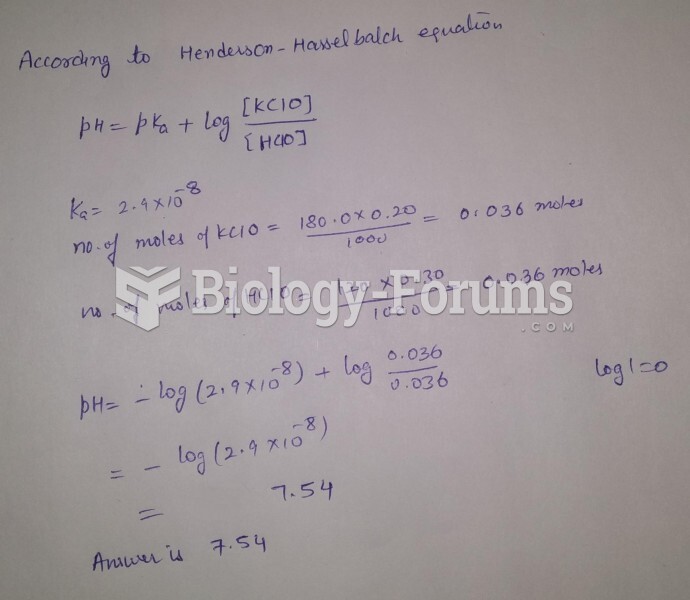
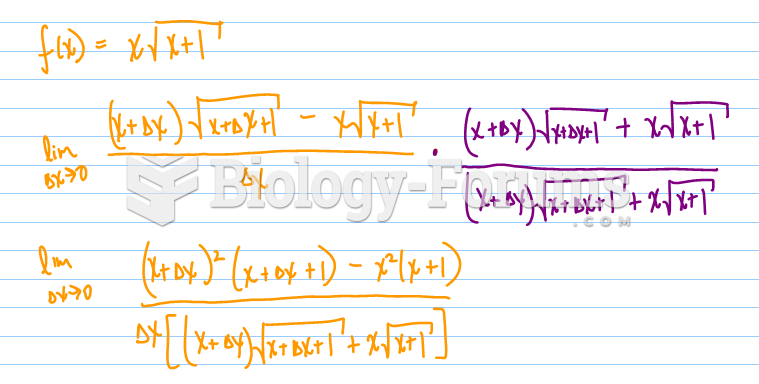|
|
|
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
The familiar sounds of your heart are made by the heart's valves as they open and close.
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.