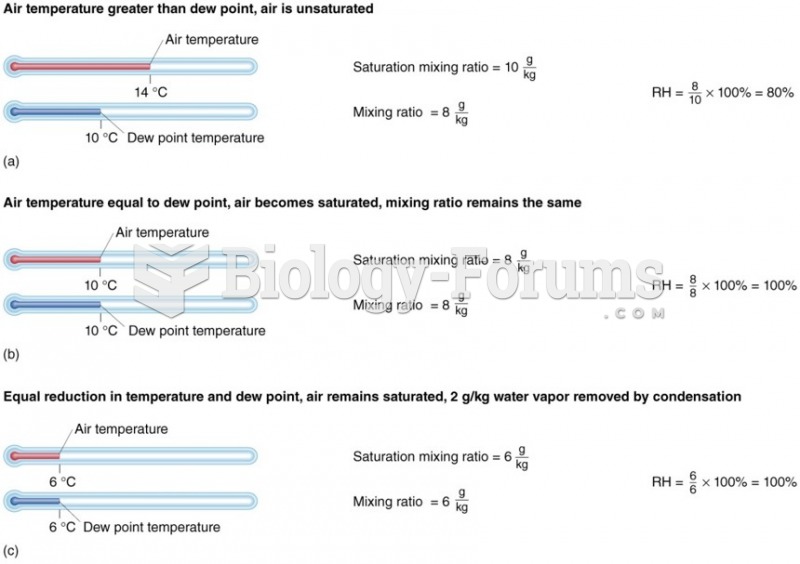
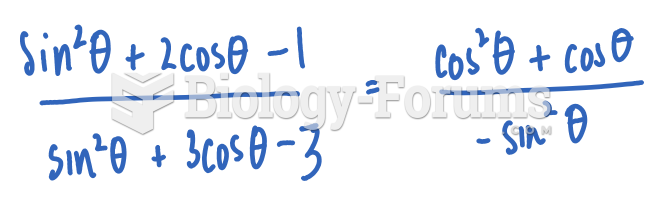This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
Did you know?
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.