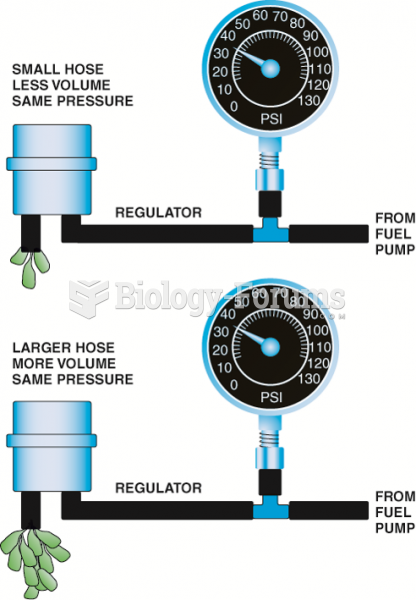
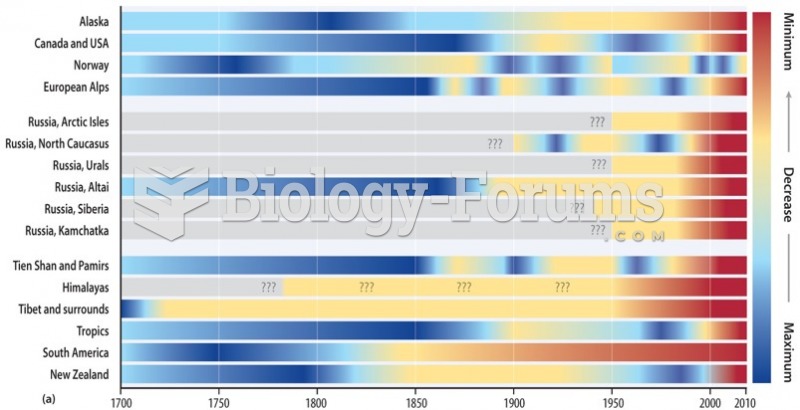
|
|
|
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
The oldest recorded age was 122. Madame Jeanne Calment was born in France in 1875 and died in 1997. She was a vegetarian and loved olive oil, port wine, and chocolate.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Vaccines cause herd immunity. If the majority of people in a community have been vaccinated against a disease, an unvaccinated person is less likely to get the disease since others are less likely to become sick from it and spread the disease.
Nearly all drugs pass into human breast milk. How often a drug is taken influences the amount of drug that will pass into the milk. Medications taken 30 to 60 minutes before breastfeeding are likely to be at peak blood levels when the baby is nursing.