This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
Did you know?
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
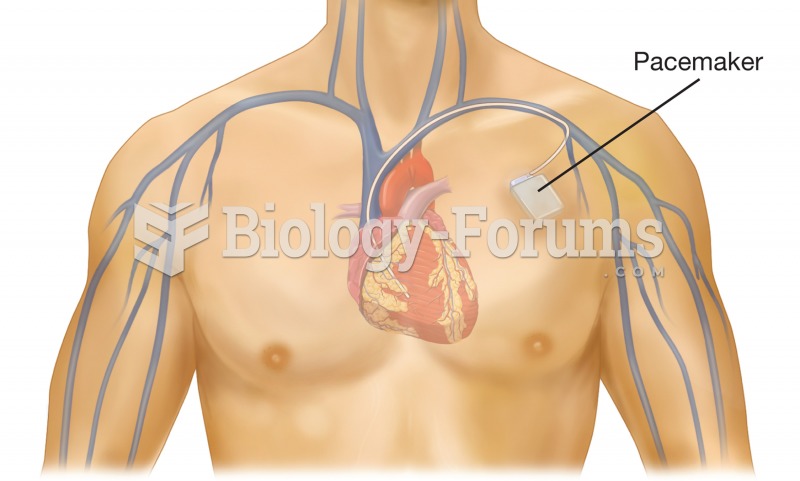
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Did you know?
If you use artificial sweeteners, such as cyclamates, your eyes may be more sensitive to light. Other factors that will make your eyes more sensitive to light include use of antibiotics, oral contraceptives, hypertension medications, diuretics, and antidiabetic medications.