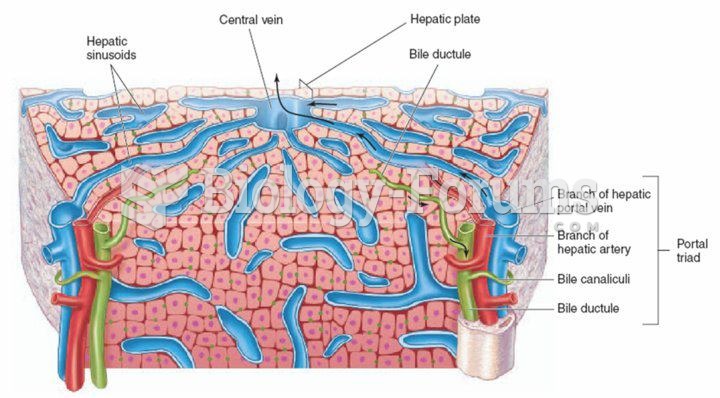
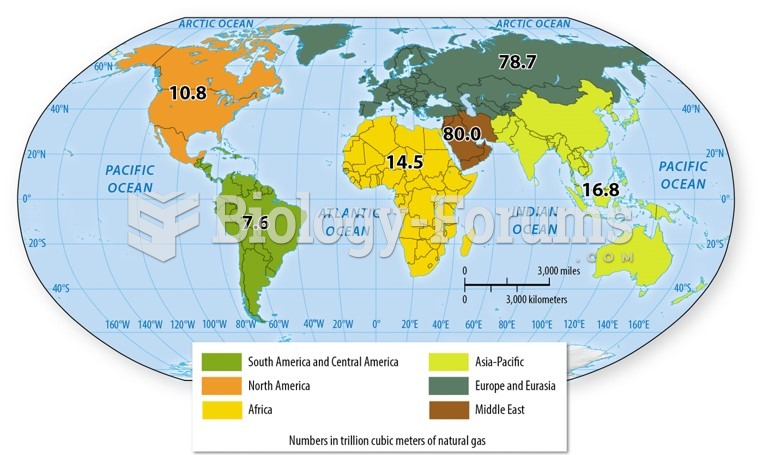
|
|
|
Fatal fungal infections may be able to resist newer antifungal drugs. Globally, fungal infections are often fatal due to the lack of access to multiple antifungals, which may be required to be utilized in combination. Single antifungals may not be enough to stop a fungal infection from causing the death of a patient.
A recent study has found that following a diet rich in berries may slow down the aging process of the brain. This diet apparently helps to keep dopamine levels much higher than are seen in normal individuals who do not eat berries as a regular part of their diet as they enter their later years.
Cyanide works by making the human body unable to use oxygen.
Approximately 25% of all reported medication errors result from some kind of name confusion.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.