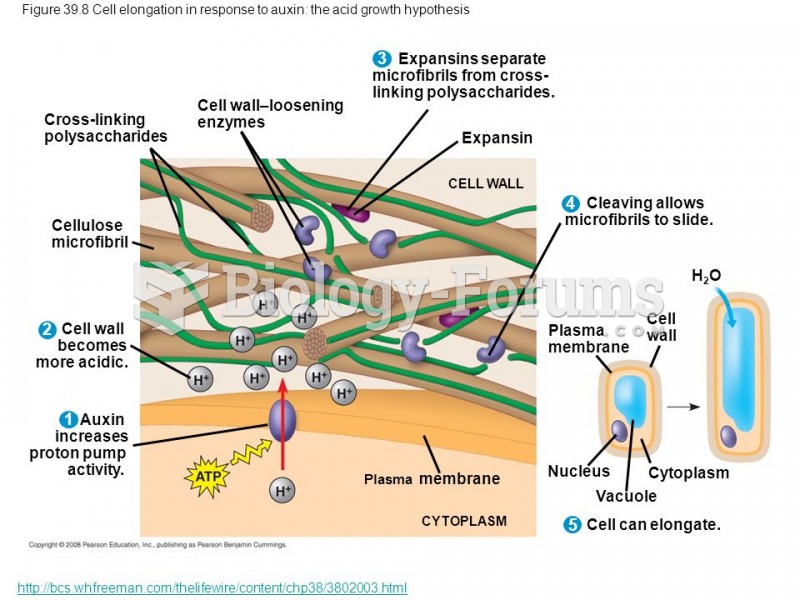
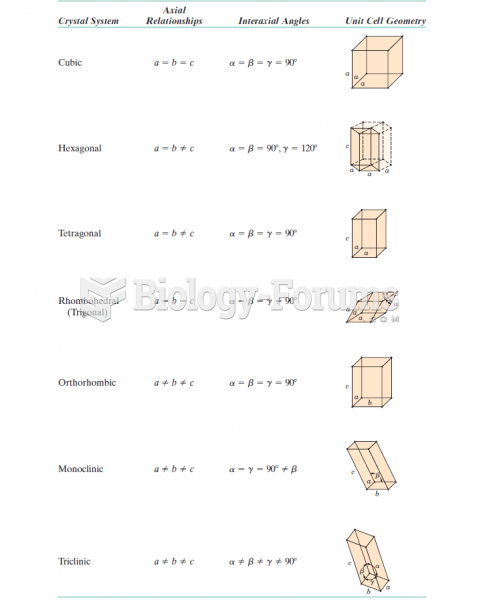
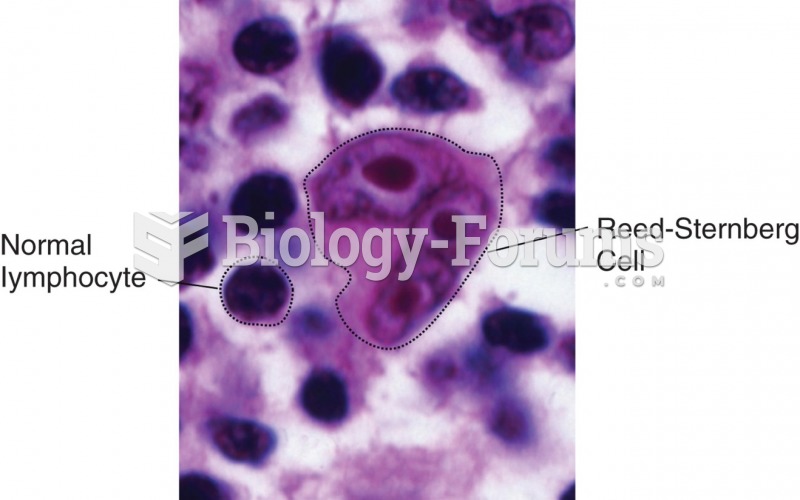
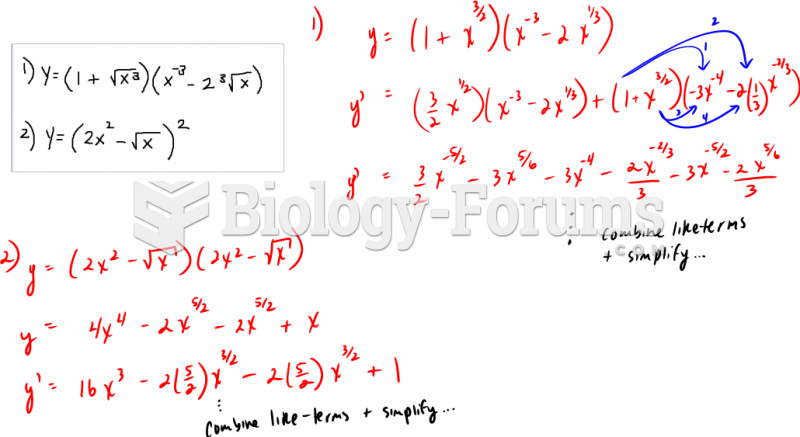
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.