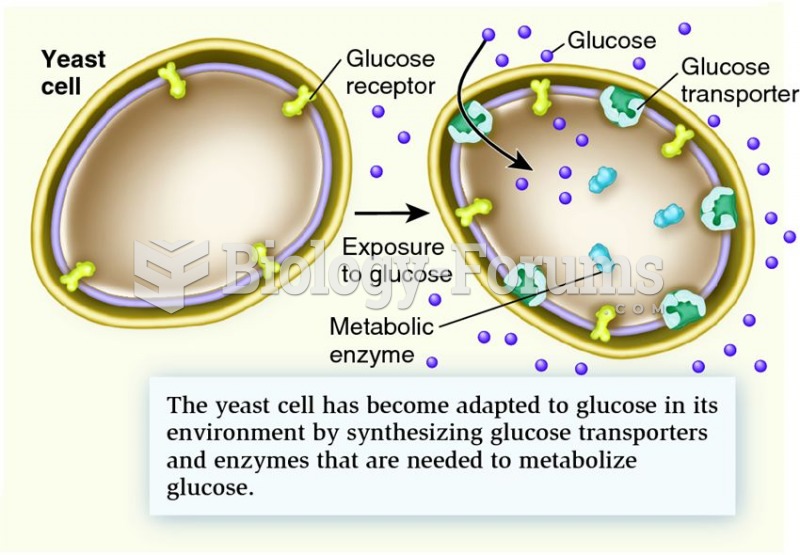
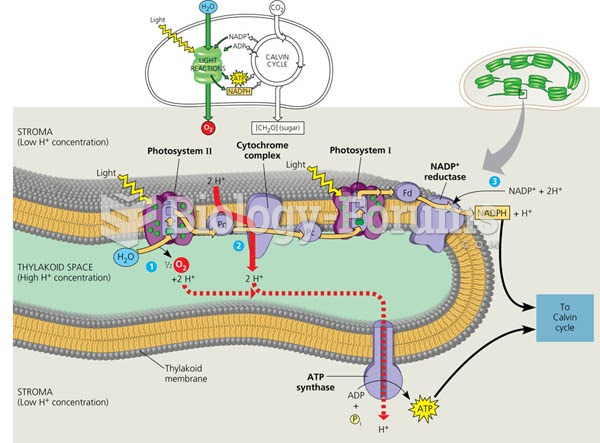
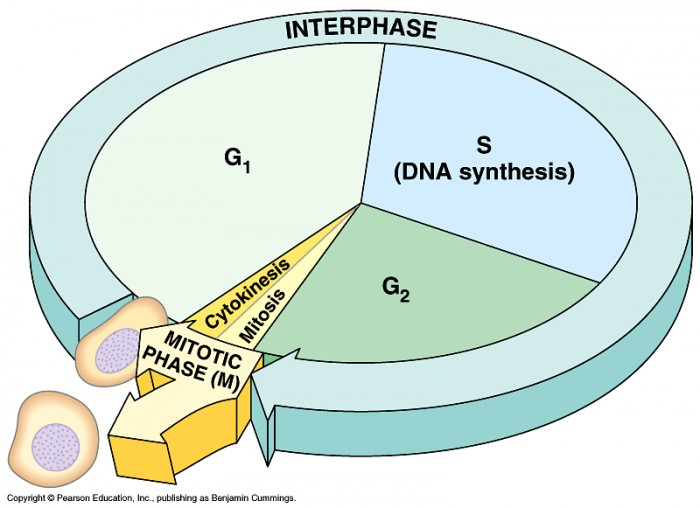
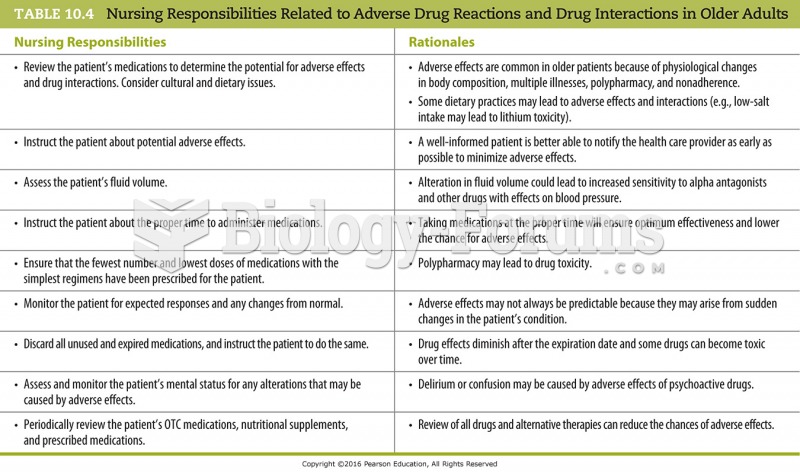
|
|
|
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.