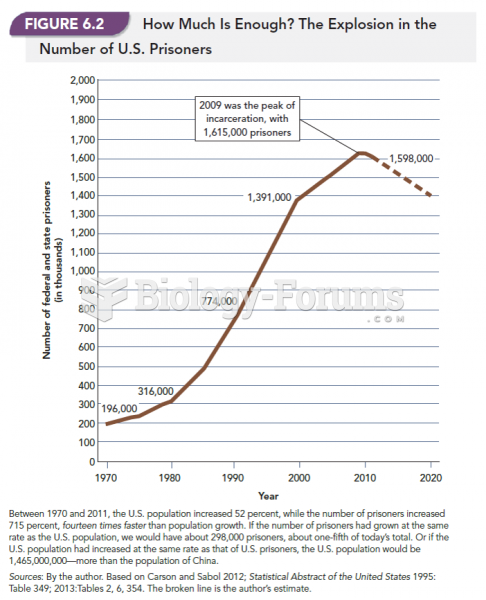
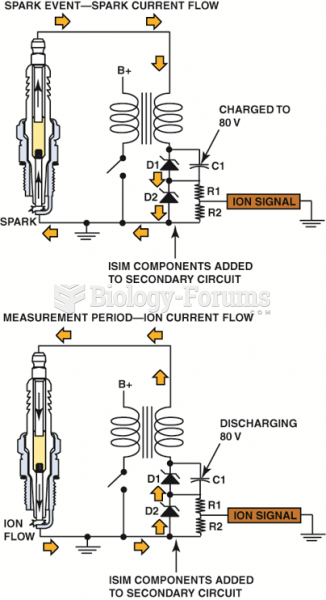
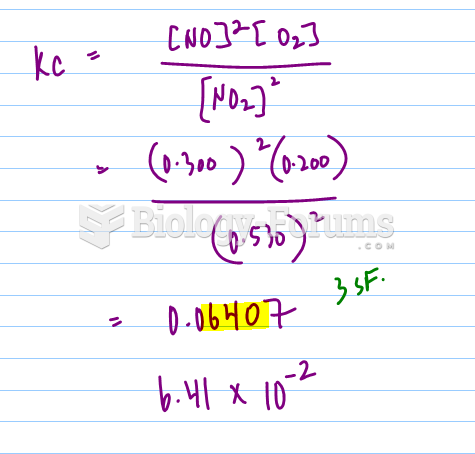
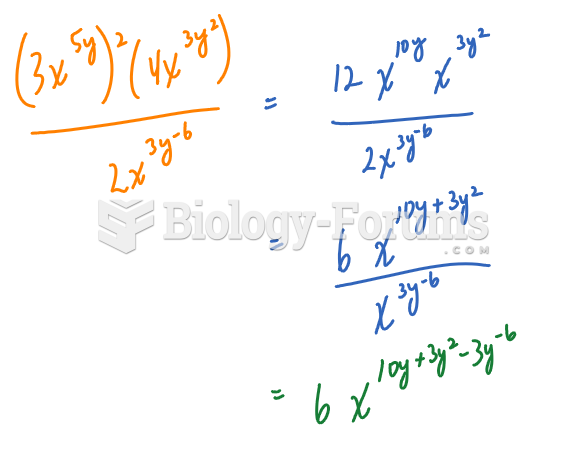|
|
|
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
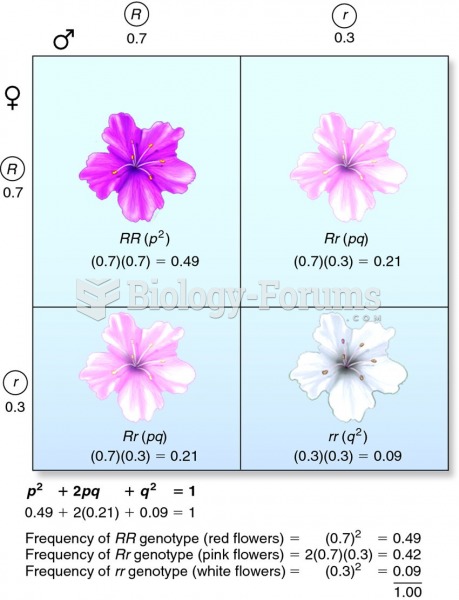 Comparing allele and genotype frequencies in a population with the Hardy-Weinberg equation and a Pun
Comparing allele and genotype frequencies in a population with the Hardy-Weinberg equation and a Pun
 A number of tree species can be interconnected by mycorrhizal fungi in the forests of British Columb
A number of tree species can be interconnected by mycorrhizal fungi in the forests of British Columb