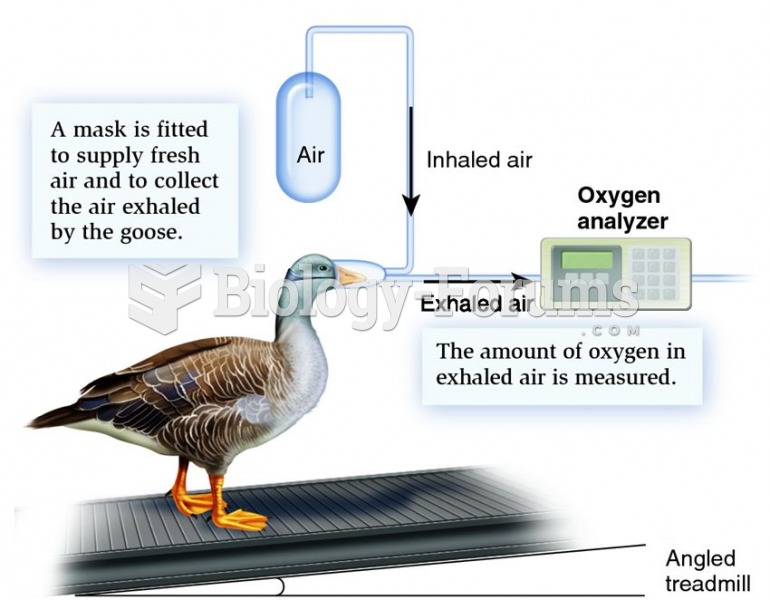
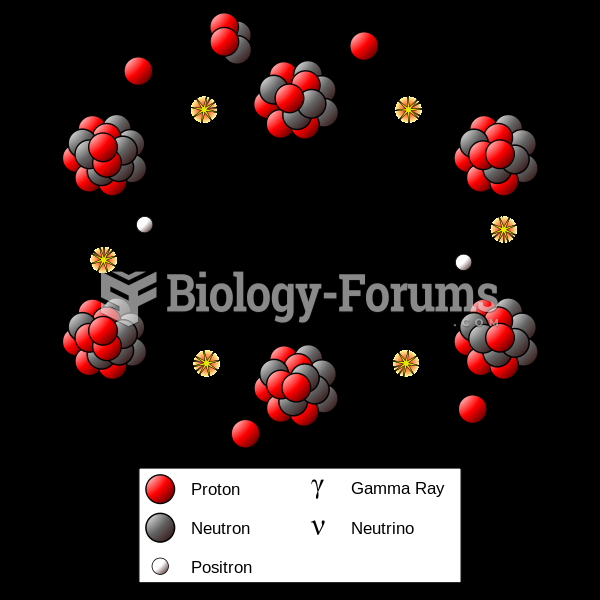
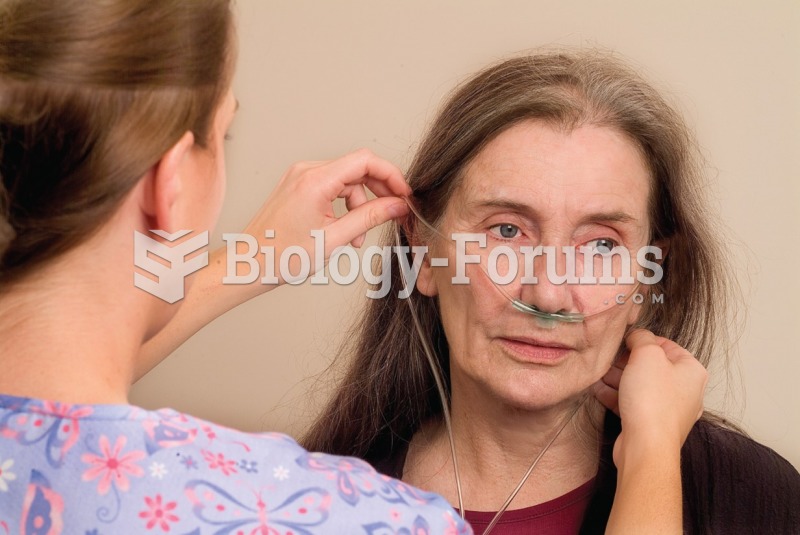
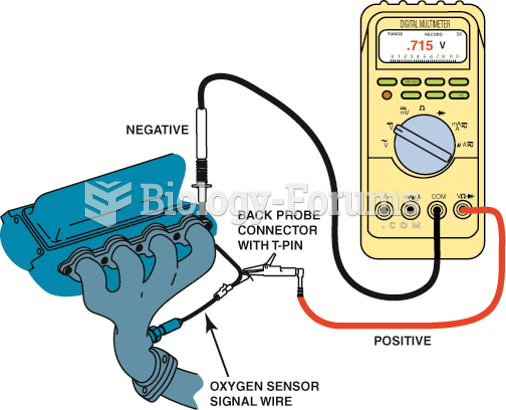
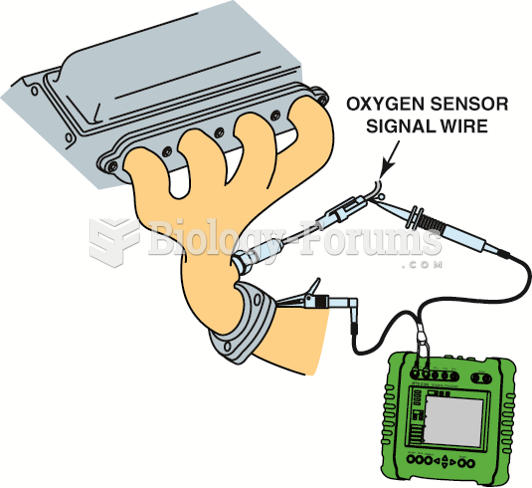
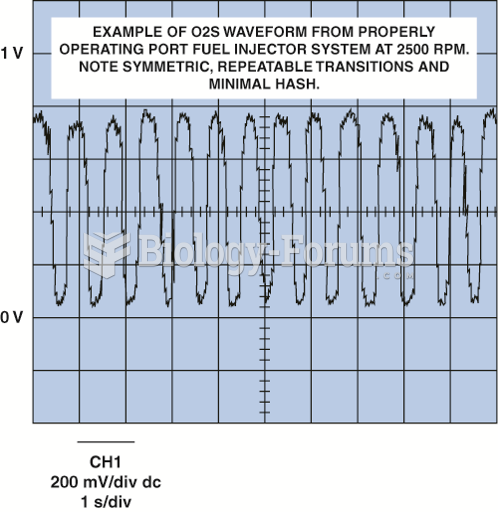
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.