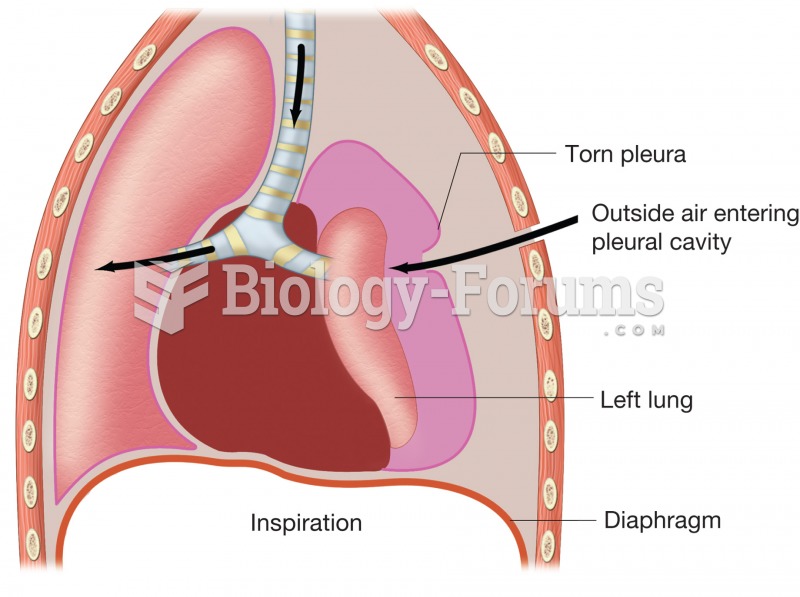
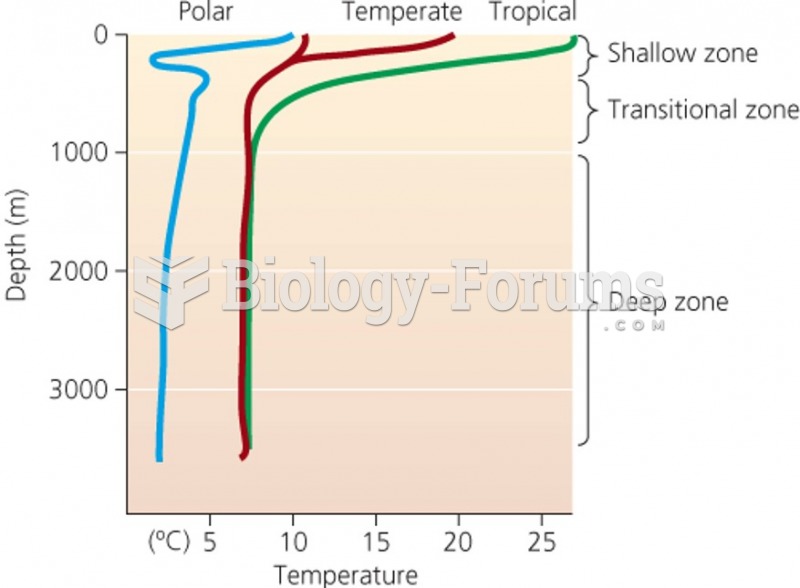
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.