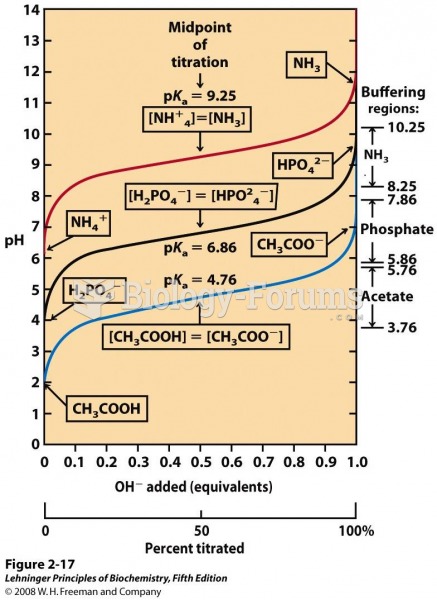
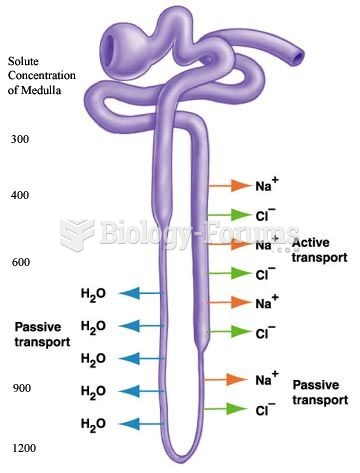
|
|
|
The familiar sounds of your heart are made by the heart's valves as they open and close.
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
The average person is easily confused by the terms pharmaceutics and pharmacology, thinking they are one and the same. Whereas pharmaceutics is the science of preparing and dispensing drugs (otherwise known as the science of pharmacy), pharmacology is the study of medications.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
About 80% of major fungal systemic infections are due to Candida albicans. Another form, Candida peritonitis, occurs most often in postoperative patients. A rare disease, Candida meningitis, may follow leukemia, kidney transplant, other immunosuppressed factors, or when suffering from Candida septicemia.