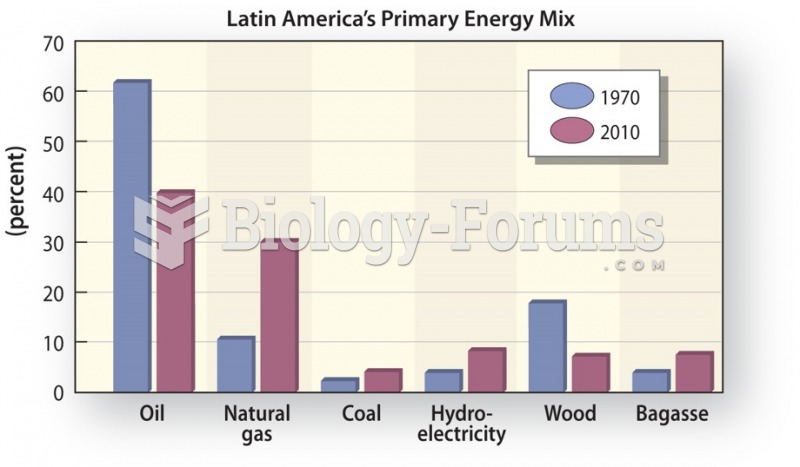
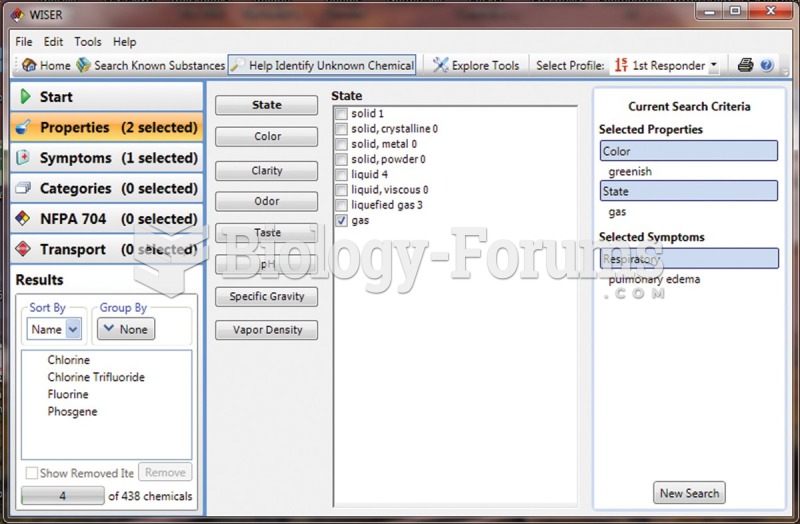
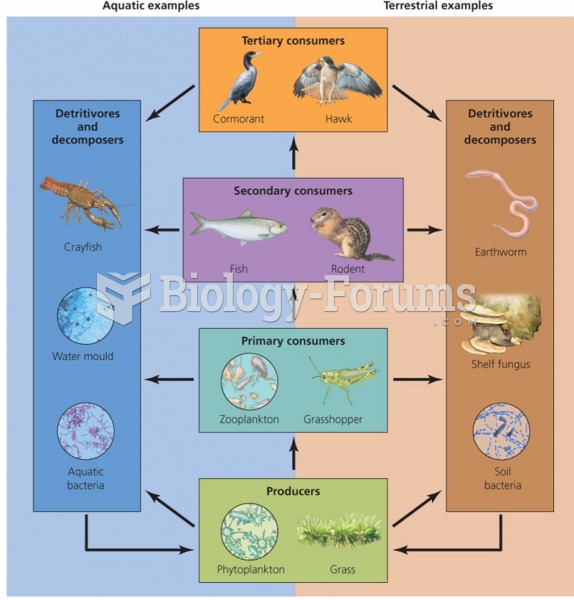
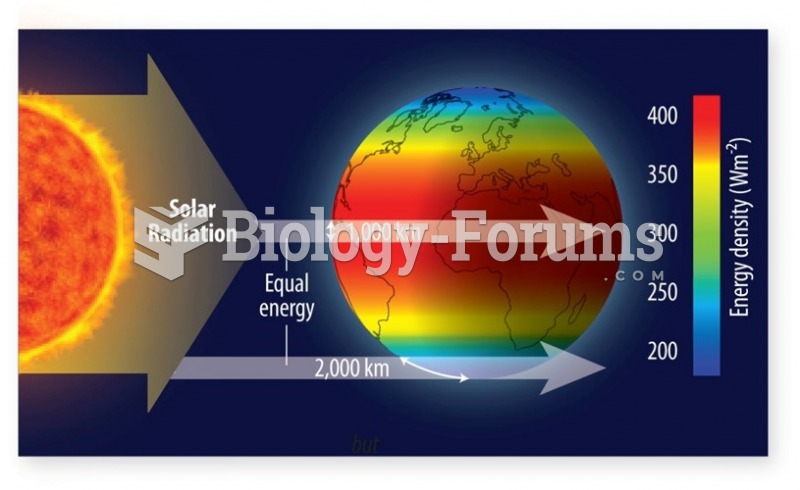
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.