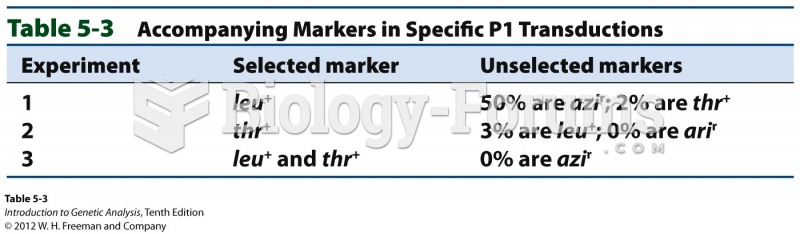
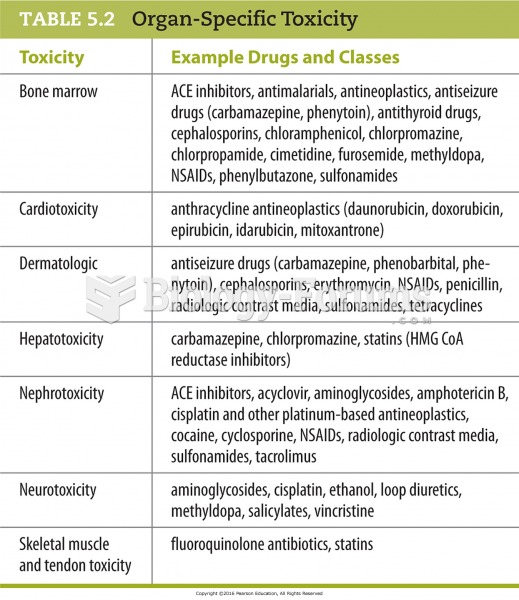
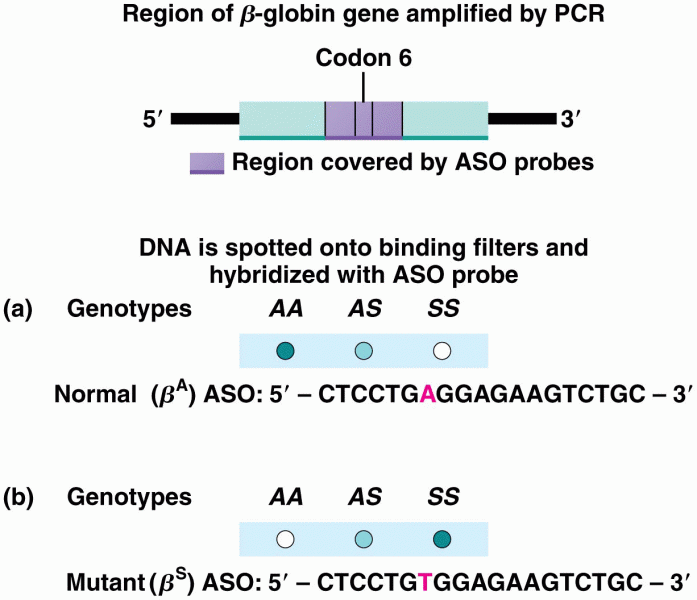
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Did you know?
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.