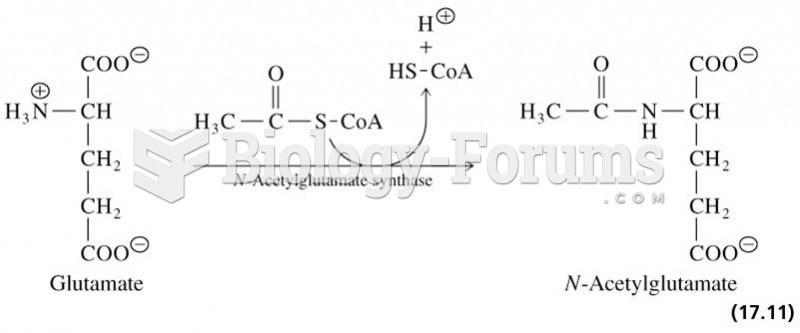
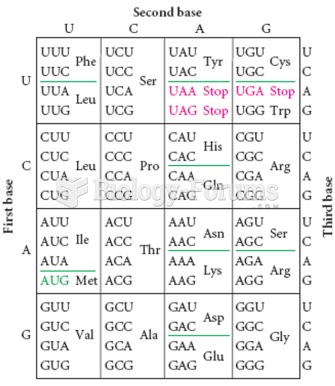
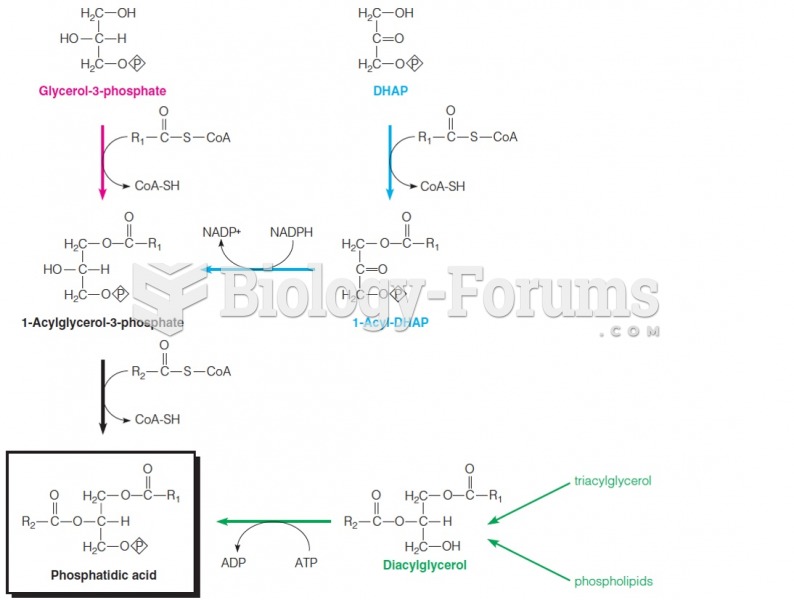
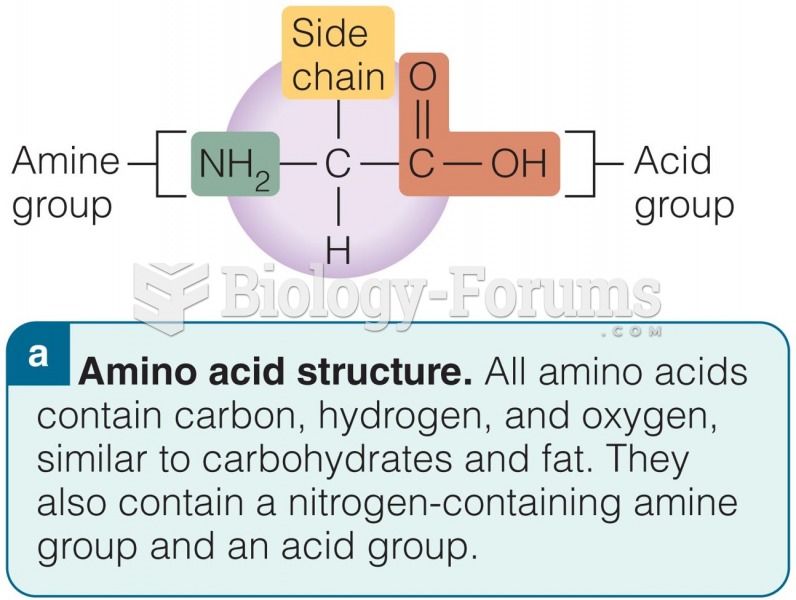
|
|
|
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
A strange skin disease referred to as Morgellons has occurred in the southern United States and in California. Symptoms include slowly healing sores, joint pain, persistent fatigue, and a sensation of things crawling through the skin. Another symptom is strange-looking, threadlike extrusions coming out of the skin.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
Vaccines prevent between 2.5 and 4 million deaths every year.