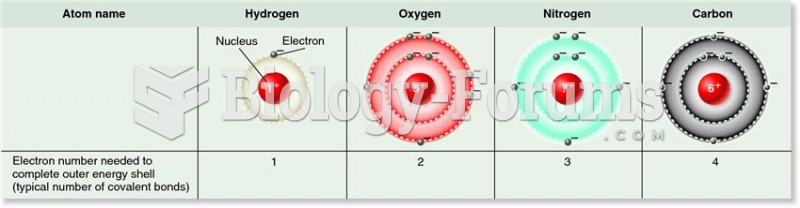
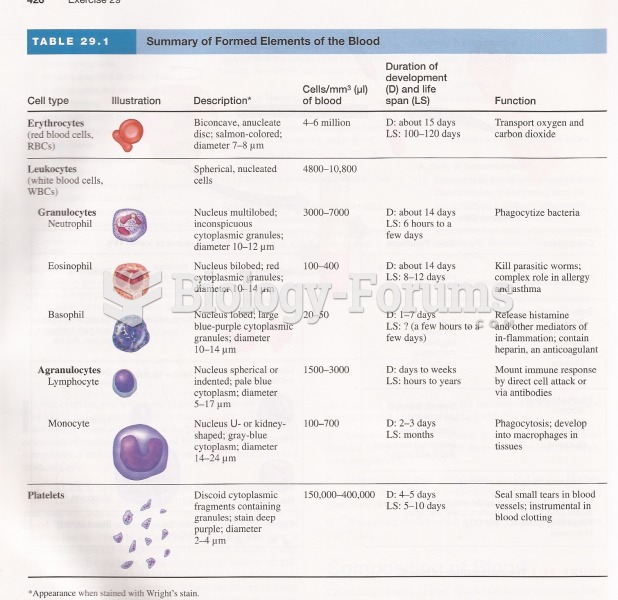
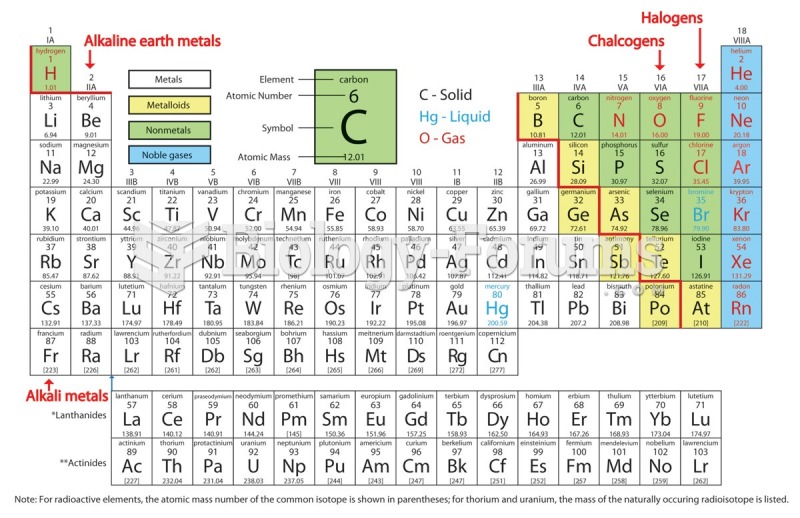
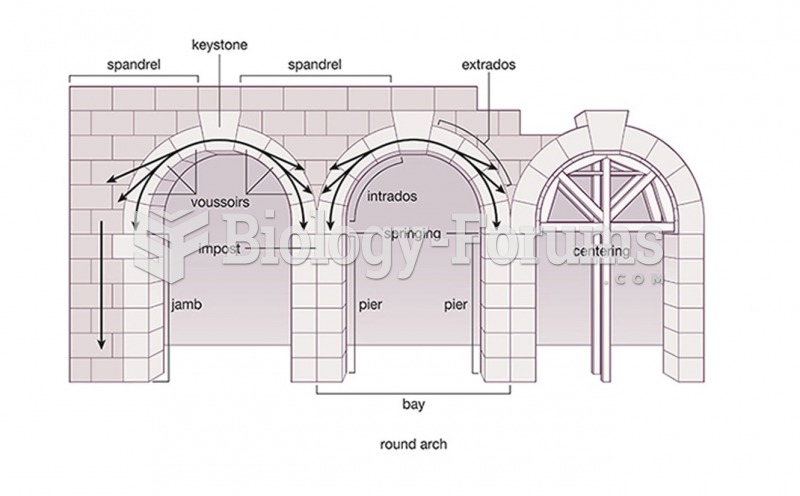
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.