This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Did you know?
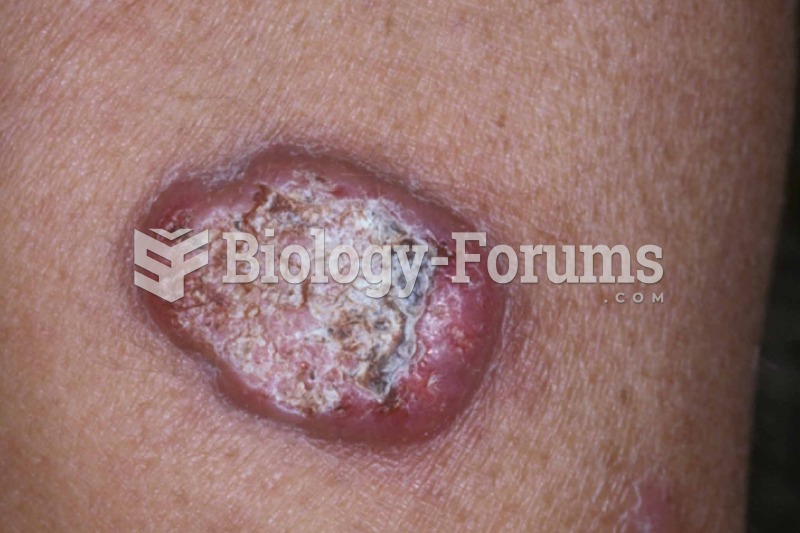
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.