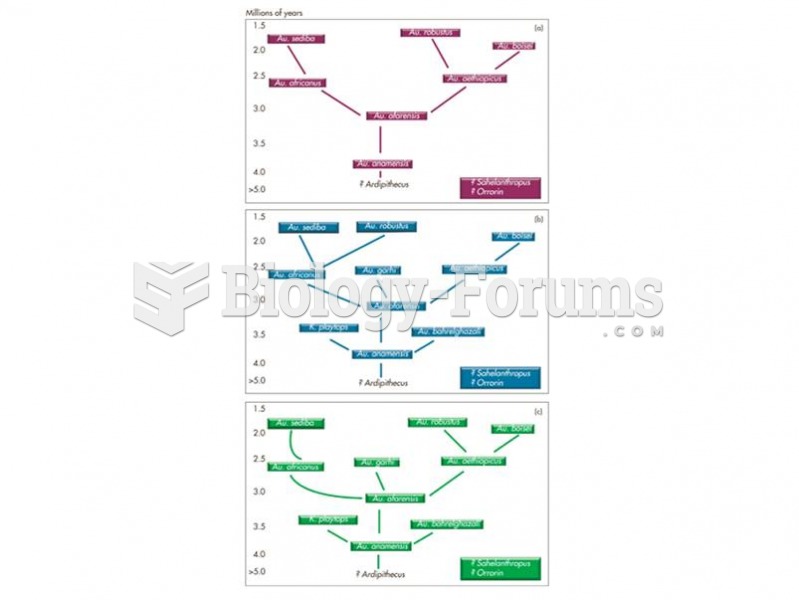
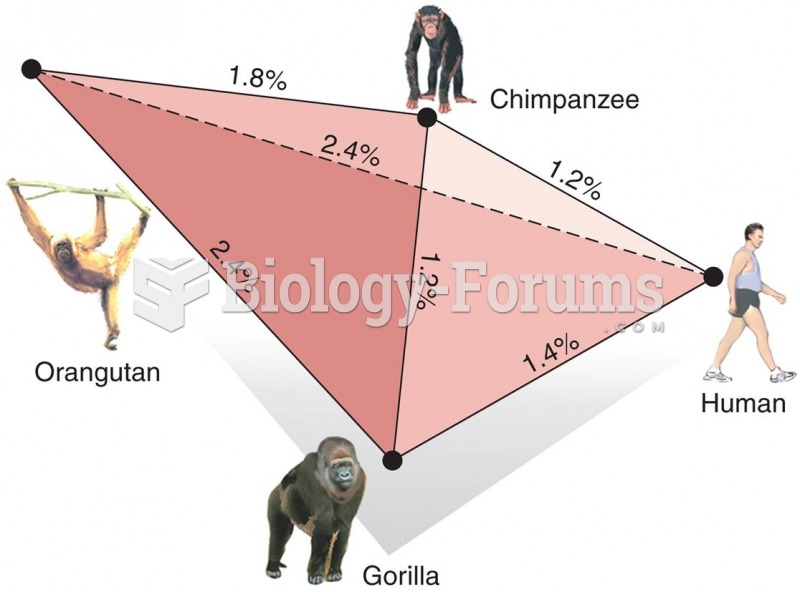
|
|
|
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Children of people with alcoholism are more inclined to drink alcohol or use hard drugs. In fact, they are 400 times more likely to use hard drugs than those who do not have a family history of alcohol addiction.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
 Several species of squirrels have melanistic phases. In large parts of United States and Canada, the
Several species of squirrels have melanistic phases. In large parts of United States and Canada, the
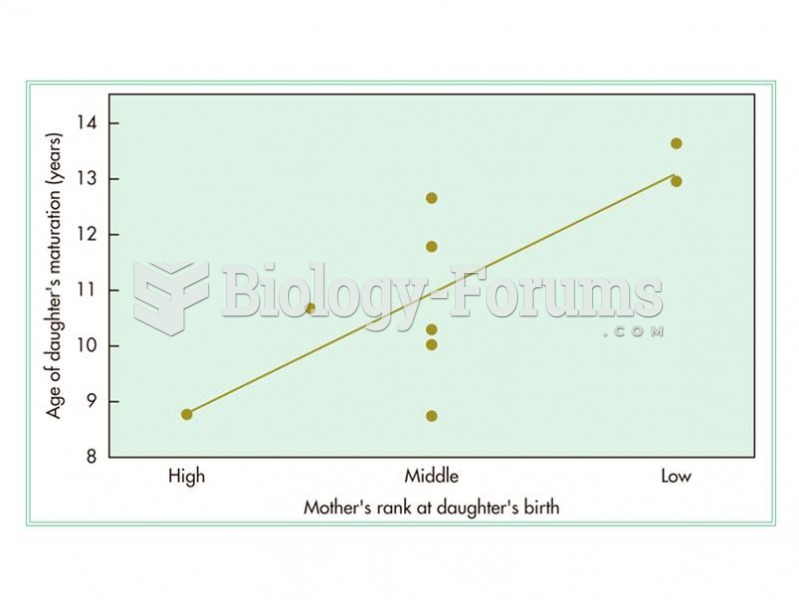 In some species, dominant females have more surviving offspring that mature earlier indicating an ad
In some species, dominant females have more surviving offspring that mature earlier indicating an ad