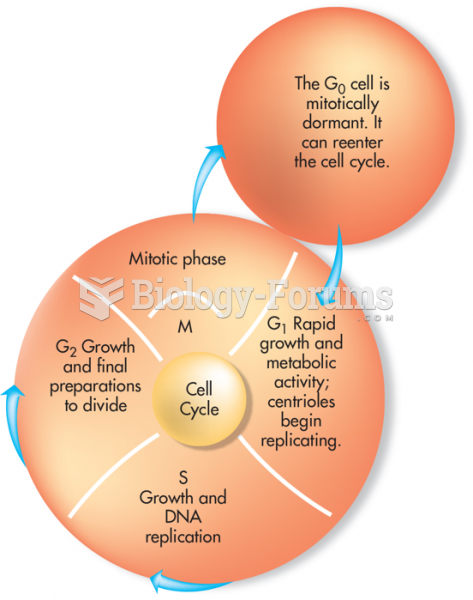
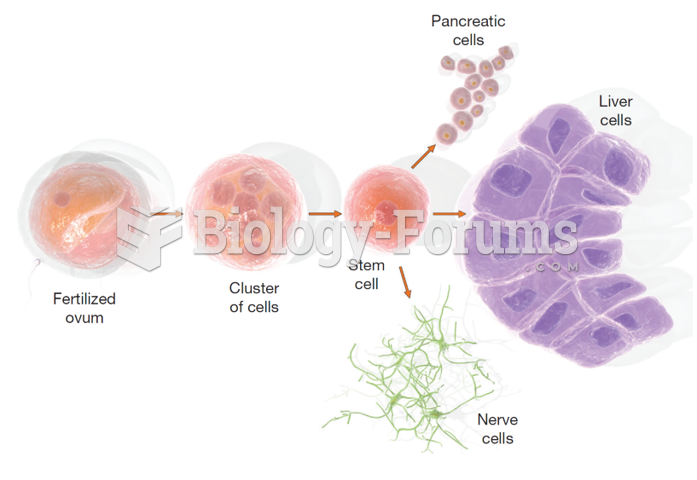
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.