This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Did you know?
All adults should have their cholesterol levels checked once every 5 years. During 2009–2010, 69.4% of Americans age 20 and older reported having their cholesterol checked within the last five years.
Did you know?
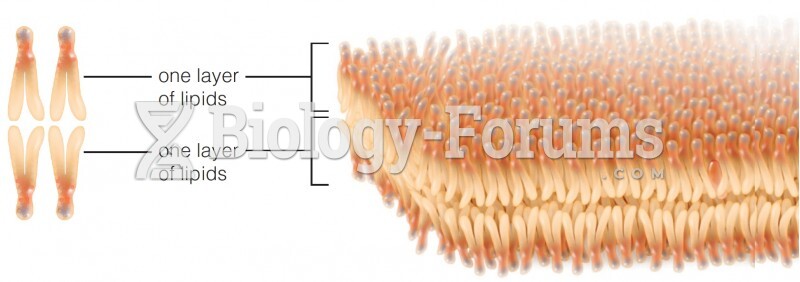
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
The longest a person has survived after a heart transplant is 24 years.