|
|
|
Bisphosphonates were first developed in the nineteenth century. They were first investigated for use in disorders of bone metabolism in the 1960s. They are now used clinically for the treatment of osteoporosis, Paget's disease, bone metastasis, multiple myeloma, and other conditions that feature bone fragility.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
The term pharmacology is derived from the Greek words pharmakon("claim, medicine, poison, or remedy") and logos ("study").
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
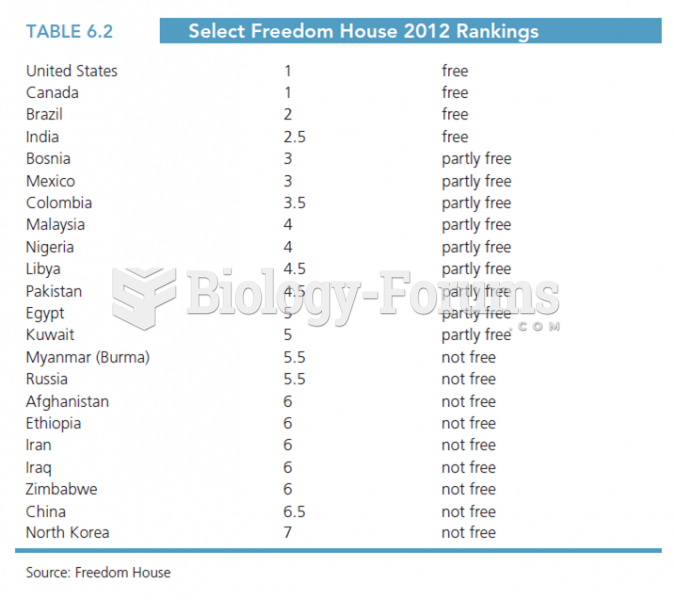 Freedom House is a nonprofit institution that uses several different factors to rank countries based
Freedom House is a nonprofit institution that uses several different factors to rank countries based
 The driver information display on a Chevrolet Impala with a 5.3 liter V-8 equipped with active fuel ...
The driver information display on a Chevrolet Impala with a 5.3 liter V-8 equipped with active fuel ...