This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Liver spots have nothing whatsoever to do with the liver. They are a type of freckles commonly seen in older adults who have been out in the sun without sufficient sunscreen.
Did you know?
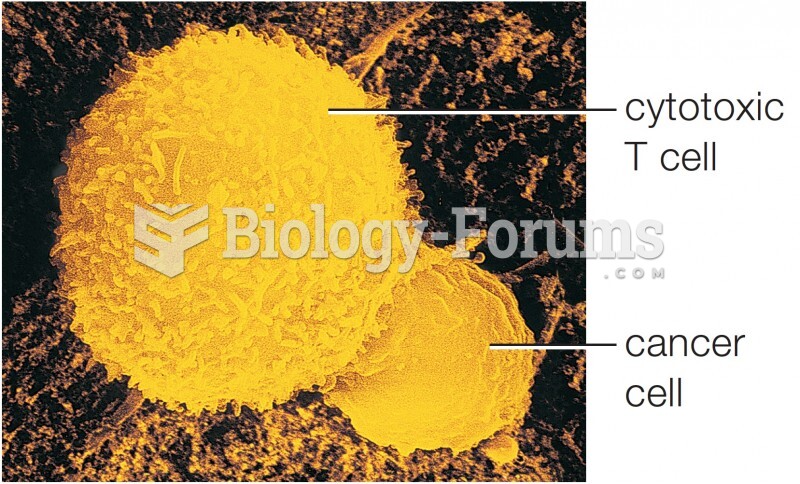
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.