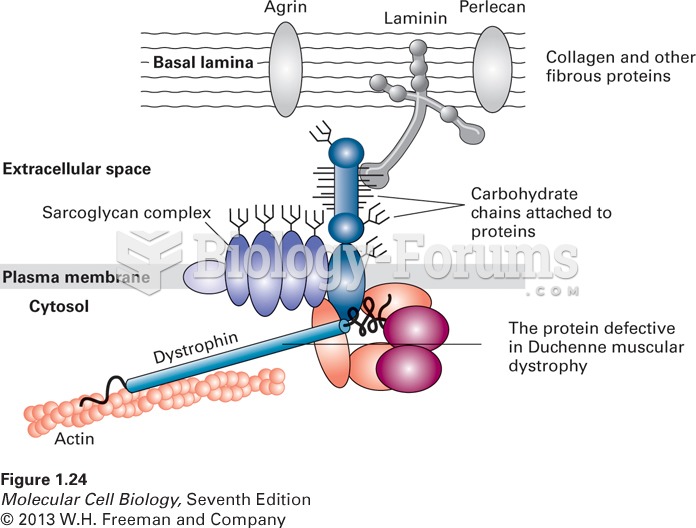
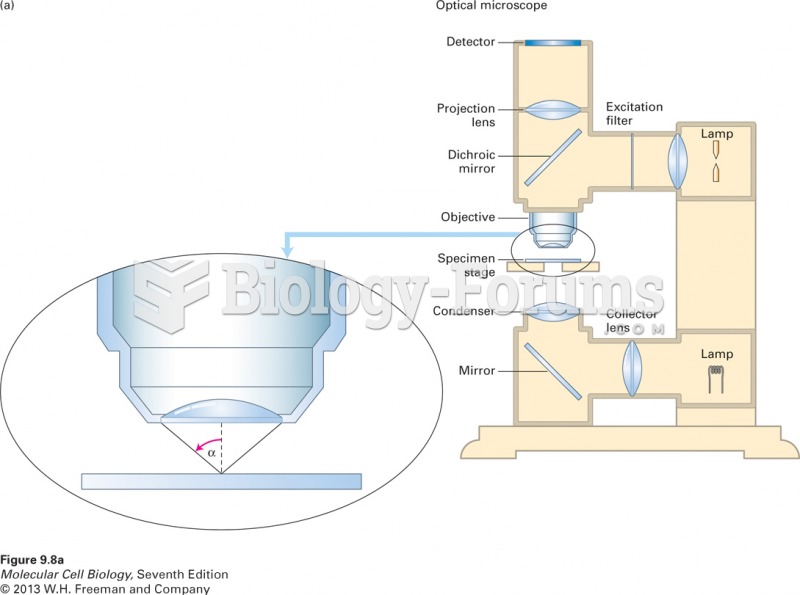
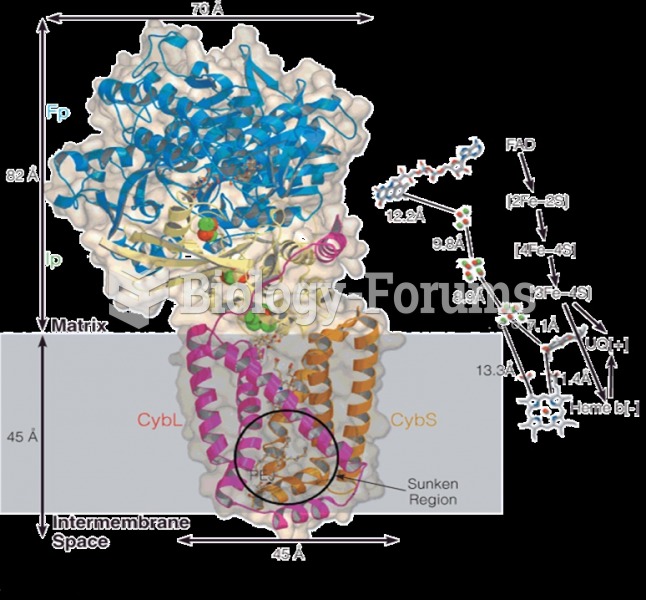
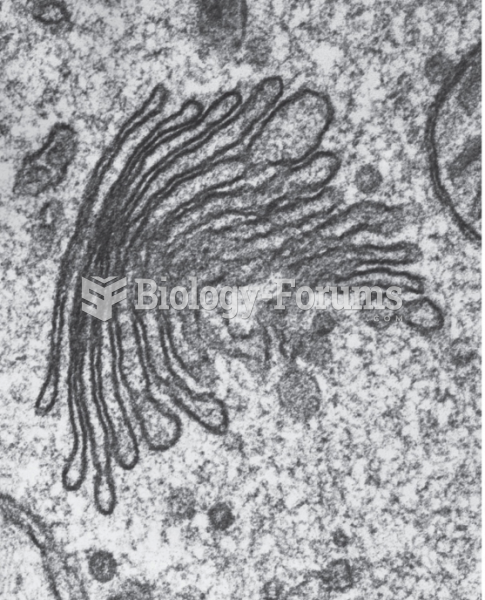
|
|
|
In 1835 it was discovered that a disease of silkworms known as muscardine could be transferred from one silkworm to another, and was caused by a fungus.
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Signs of depression include feeling sad most of the time for 2 weeks or longer; loss of interest in things normally enjoyed; lack of energy; sleep and appetite disturbances; weight changes; feelings of hopelessness, helplessness, or worthlessness; an inability to make decisions; and thoughts of death and suicide.