|
|
|
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
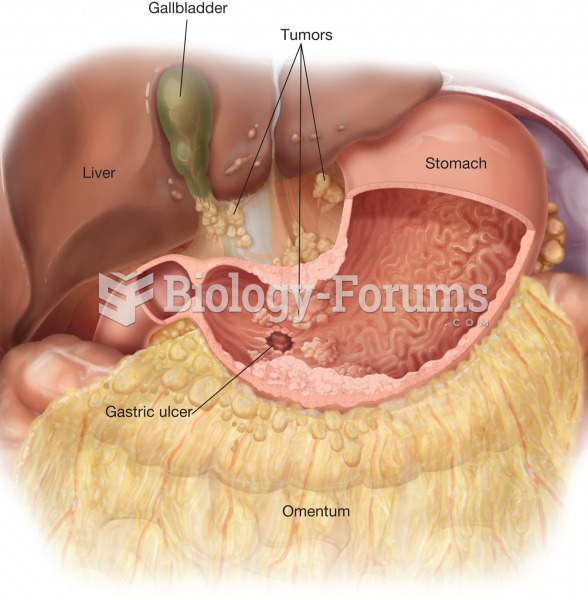
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
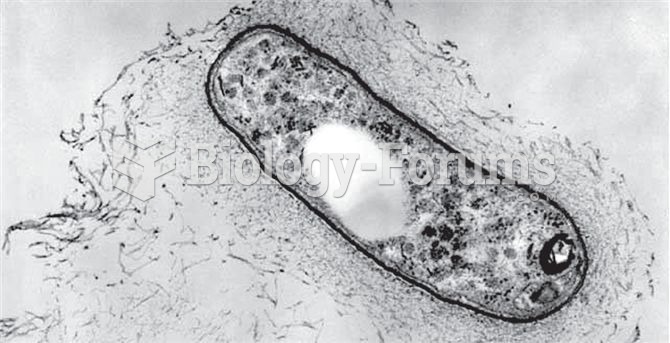
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
 Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
 Several species of squirrels have melanistic phases. In large parts of United States and Canada, the
Several species of squirrels have melanistic phases. In large parts of United States and Canada, the