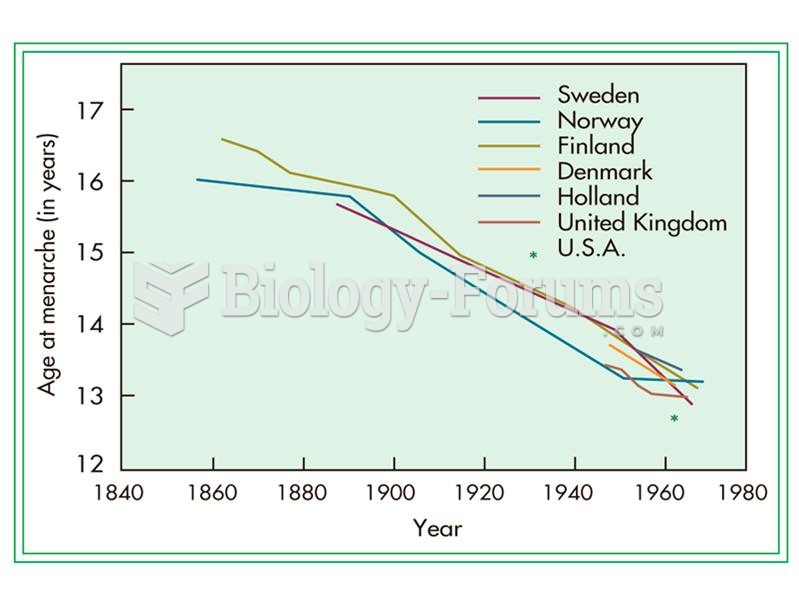
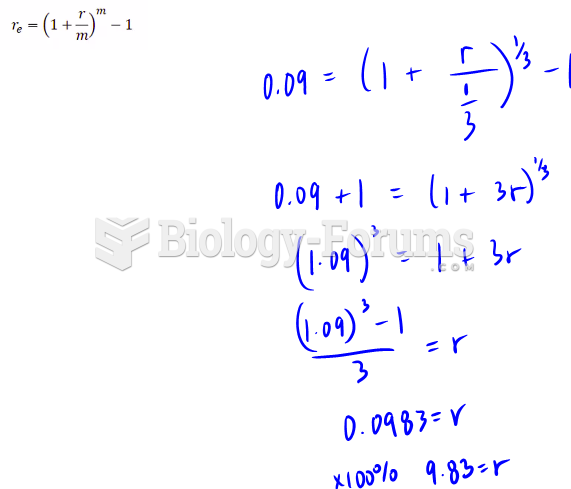|
|
|
There are approximately 3 million unintended pregnancies in the United States each year.
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.