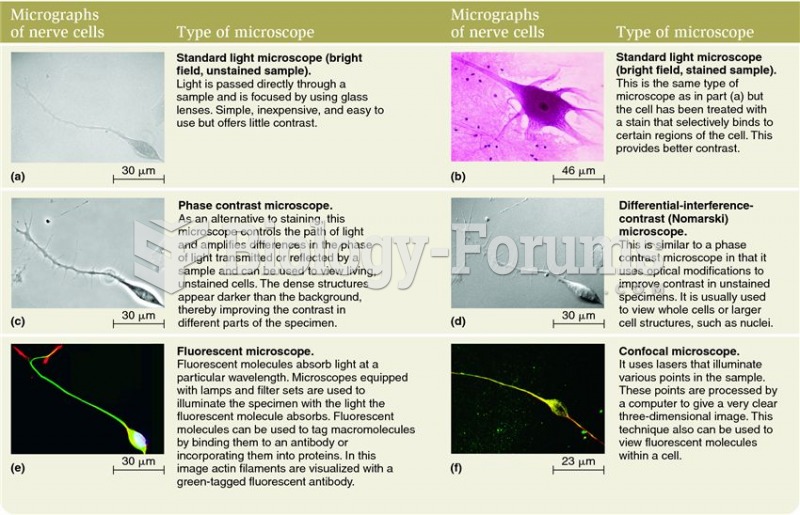
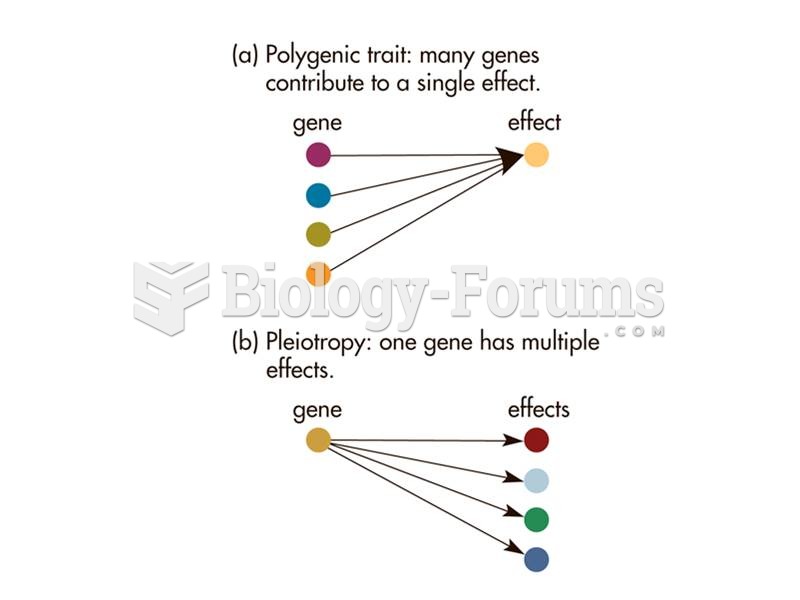
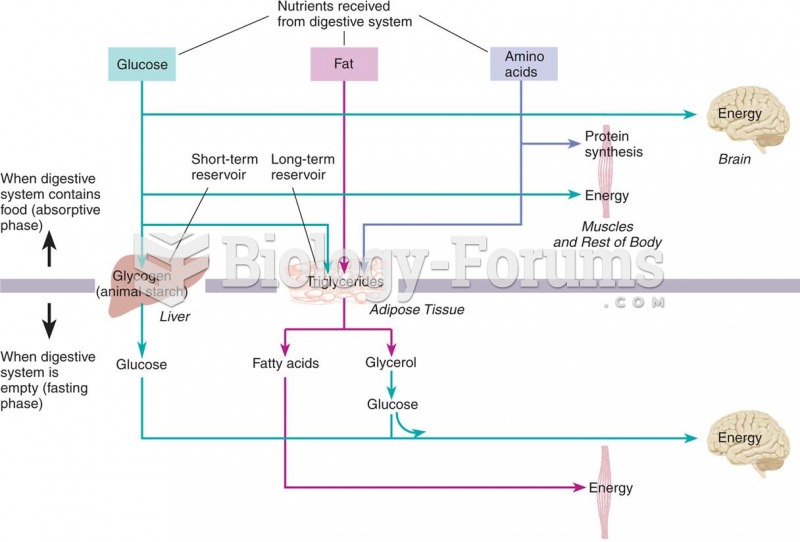
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
Interferon was scarce and expensive until 1980, when the interferon gene was inserted into bacteria using recombinant DNA technology, allowing for mass cultivation and purification from bacterial cultures.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.