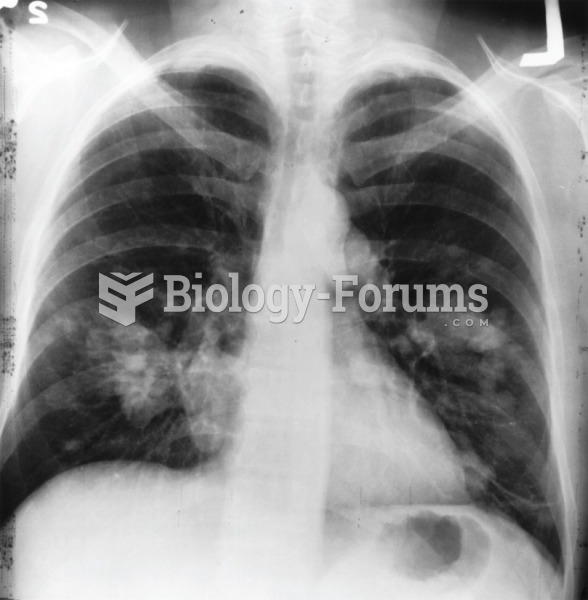
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
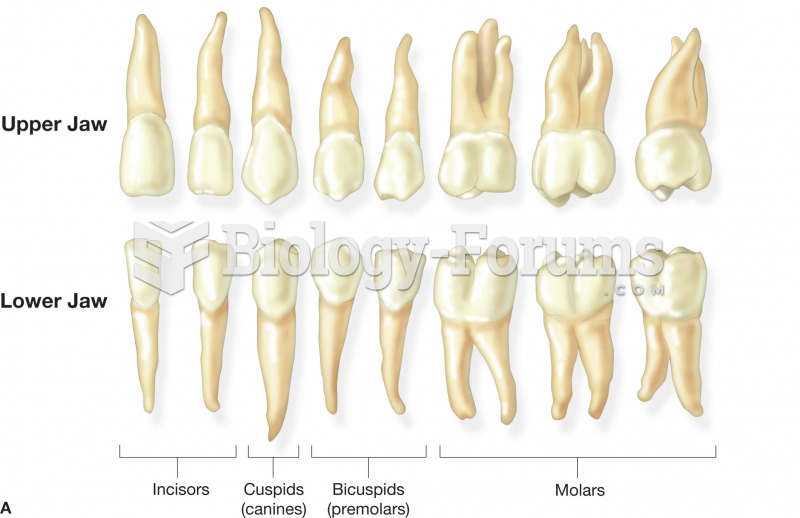 (A) The name and shape of the adult teeth. These teeth represent those found in the right side of th
(A) The name and shape of the adult teeth. These teeth represent those found in the right side of th
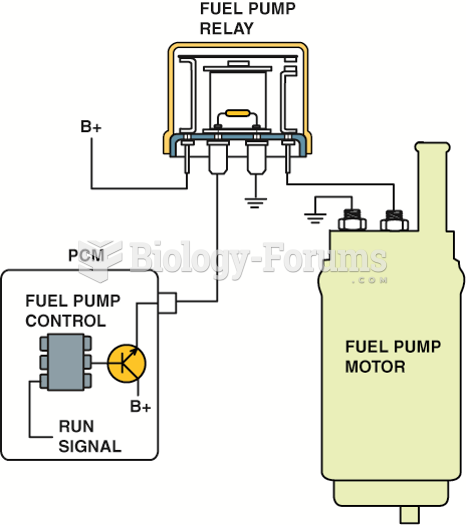 A typical module-controlled high-side driver (HSD) where the module itself supplies the electrical ...
A typical module-controlled high-side driver (HSD) where the module itself supplies the electrical ...