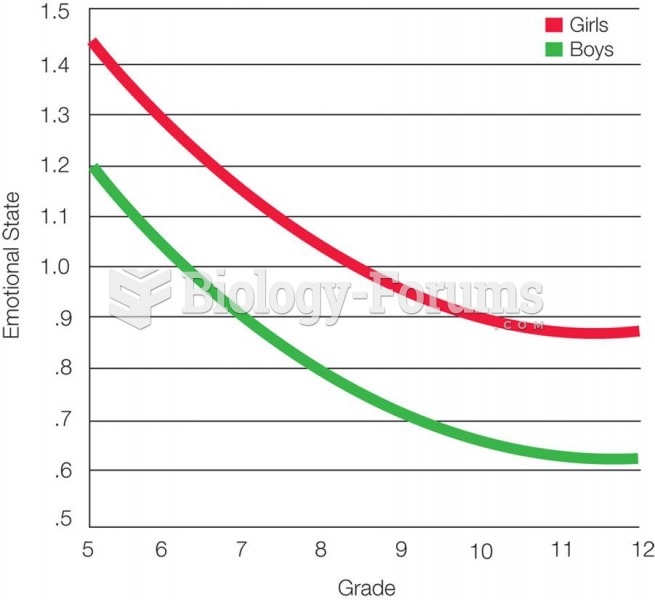
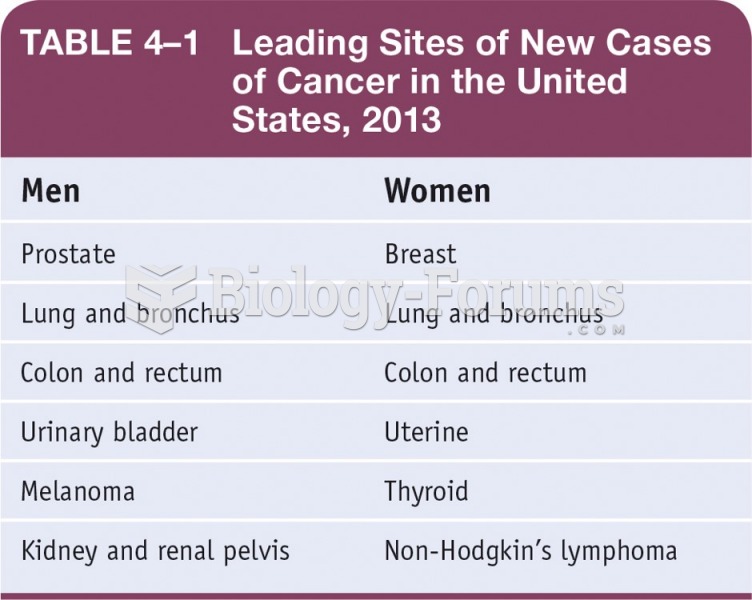
|
|
|
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Prostaglandins were first isolated from human semen in Sweden in the 1930s. They were so named because the researcher thought that they came from the prostate gland. In fact, prostaglandins exist and are synthesized in almost every cell of the body.
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
Though the United States has largely rejected the metric system, it is used for currency, as in 100 pennies = 1 dollar. Previously, the British currency system was used, with measurements such as 12 pence to the shilling, and 20 shillings to the pound.
 A woman in a net on a Congo shore. Although the importation of slaves was illegal after 1807, histor
A woman in a net on a Congo shore. Although the importation of slaves was illegal after 1807, histor
 In 2008, Barack Obama was elected president of the United States, the first minority to achieve this ...
In 2008, Barack Obama was elected president of the United States, the first minority to achieve this ...